21: Aromaticity
- Page ID
- 143108
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)MO Theory of Aromatic and Anti-Aromatic Compounds
Hückel MO diagrams for cyclic conjugated systems:
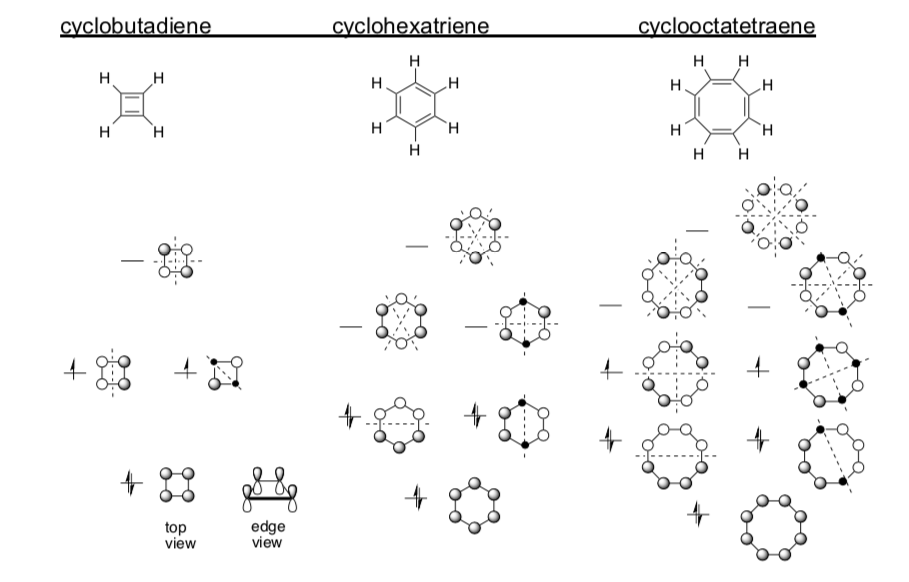
Again, look for patterns:
-
What happens to energy level (wavelength) of the lowest orbital as the conjugated system gets longer?
-
The number of energy levels for each compound is ___________to the number of p orbitals in each orbital picture.
-
When are there degenerate (equivalent energy) orbitals?
-
The number of pi electrons for each compound is equal to the _______per pi bond and _______ per aligned lone pair and _______ per cation.
-
How many nodes per each level? Is there a pattern to the number of nodes?
-
In which structures are there non-bonding levels?
-
When are there unpaired electrons?
Using the patterns that you found on the previous page,
-
Fill in the missing electrons and MO labels for cyclodecapentaene, cyclododecahexaene & cyclotetradecaheptaene
Cyclobutadiene is particularly unstable, “anti-aromatic”.
Cyclohexatriene (benzene) is “aromatic” – exceptionally stable.
-
Predict which of the other compounds will be aromatic and which will be anti-aromatic (circle one).
cyclooctatetraene: aromatic anti-aromatic
cyclodecapentaene: aromatic anti-aromatic
cyclododencahexaene: aromatic anti-aromatic
cyclotetradecaheptaene: aromatic anti-aromatic
-
How many pairs of electrons pairs in the aromatic molecules?
-
How many pairs of electrons pairs in the anti-aromatic molecules?
Another way to predict aromaticity or anti-aromaticity is to use Hückel’s rule:
Aromatic systems have 4n+2 p electrons, where n is any integer.
- Does a molecule with 42 p electrons fit Hückel’s rule? 42 = 4n + 2; what is n?
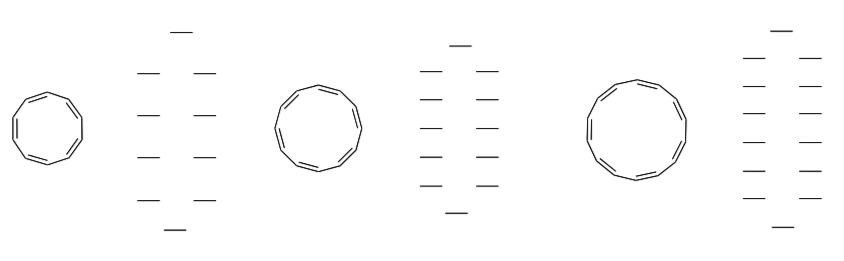
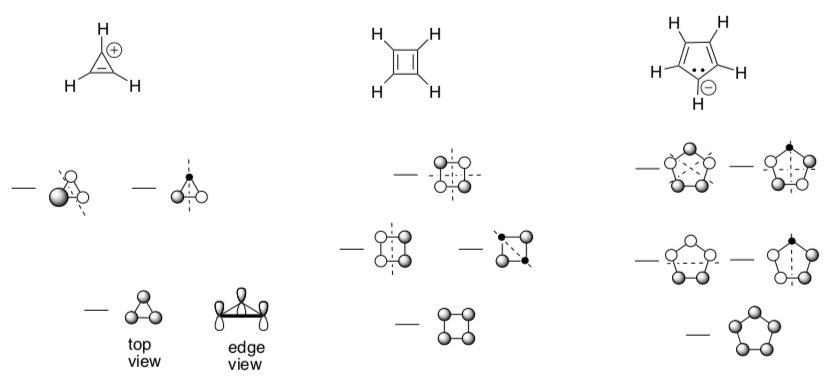
So far we have drawn the MOs for even numbered systems such as 1,3- cyclobutadiene and benzene. However, there are numerous examples that have odd numbered rings.
Look at the 3-, 4- and 5-membered rings:
-
Fill in the electrons in each of the MO diagrams.
Again, look for patterns:
-
How does the number of molecular orbitals for one compound compare to the number of p orbitals in each orbital picture?
-
How many energy levels are there for each compound? When are there degenerate (equivalent energy) orbitals?
-
How many nodes for each level?
-
When are there non-bonding levels?
-
What is different about orbital diagrams for the odd membered rings vs. the even membered ring structures?
-
Predict which of these compounds will be aromatic and which will be anti- aromatic (circle one).
cyclopropenyl cation: aromatic anti-aromatic
cyclopentadienyl anion: aromatic anti-aromatic
Rules for Aromaticity and Anti-aromaticity
Aromatic and anti-aromatic molecules BOTH have similar structural requirements. They only differ in the number of p electrons (and hence their MO diagrams and stability).
-
List the rules for Aromaticity/Anti-aromatic compounds.
-
Benzene is aromatic but 1,3 butadiene is anti-aromatic

Aromatic compounds must have ______________ electrons.
Anti-aromatic compounds must have ______________ electrons.
-
Benzene is aromatic but hexatriene is not aromatic and is less stable.

Aromatic and Anti-aromatic compounds must be ______________.
-
Benzene is aromatic but cycloheptatriene is not aromatic and is less table

Aromatic and Anti-aromatic compounds must be ______________.
-
The two molecules below do not exhibit aromatic properties:
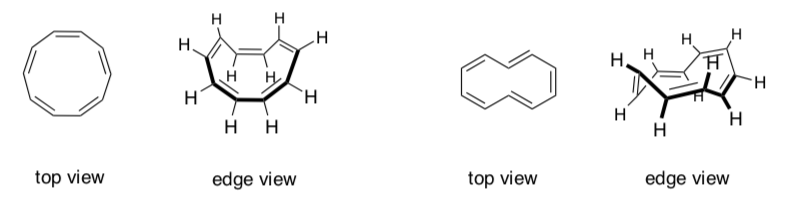
Aromatic and Anti-aromatic compounds must be ______________.
Any molecule that doesn’t fit these rules is non-aromatic.
-
Stability
Aromaticity is a chemical property describing the way in which a conjugated ring is more stable than would be expected by the stabilization of conjugation alone. Anti-aromatic is more unstable that would be predicted.
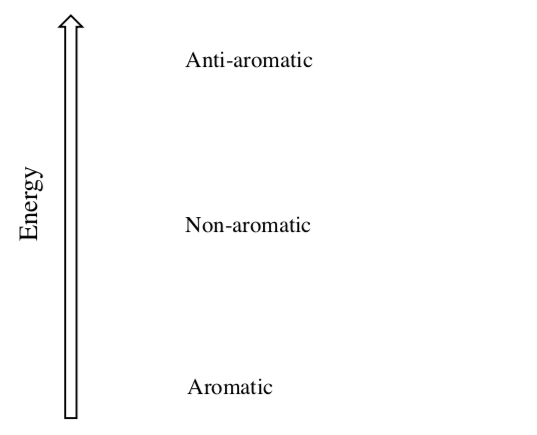
-
Most compounds prefer to be ____________ (low/high energy).
-
If an anti-aromatic molecule can twist so that the p orbitals are no longer aligned, then the molecule will become _________________ which is (lower/higher) in energy.
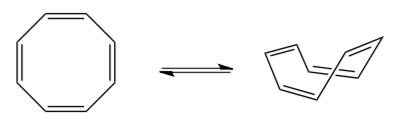
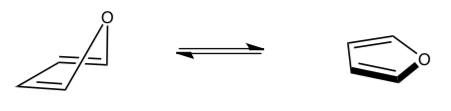
-
If a non-aromatic structure can re-hybridize one atom so that a lone pair is in an aligned orbital, then the molecule will become _________________ which is (lower/higher) in energy.
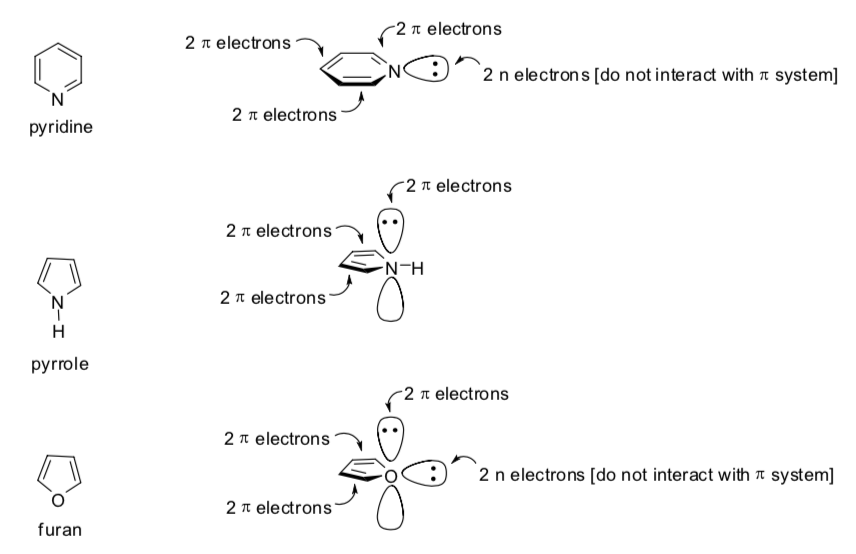
Heteroaromatic Compounds
There are two rules for rings with heteroatoms (any atom that isn’t C or H).
-
An atom with a lone pair can become sp2 hybridized if it will increase the conjugation.
-
Any atom can only contribute one p orbital (maximum 2 electrons to the aromatic structure).
Here are three rings with heteroatoms:
-
Draw in the p orbitals that participate in the conjugation.
-
Indicate whether the lone pair is in an orbital that is aligned with the conjugation or not.
-
Are these rings aromatic?
Frost Circles
Frost Circles are a mnemonic to remember the pattern of aromatic type orbitals.
Rules:
-
The aromatic/anti-aromatic molecule is drawn point down.
-
A circle is drawn around the compound.
-
Every point on the ring represents an orbital level.
A Frost Circle for 1,3-butadiene is shown below:
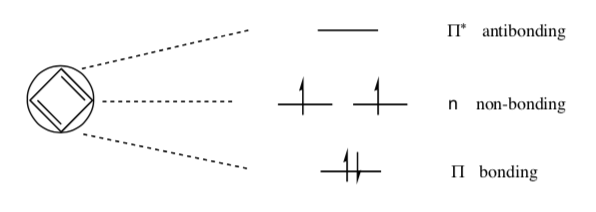
-
Use a Frost Circle to predict the orbital levels for cycloheptatrienyl anion.
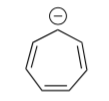
-
Fill the electrons in the orbitals.
-
Predict whether cycloheptatrienyl anion is aromatic or anti-aromatic.
Summary of Aromaticity
-
Develop a concept map for “non-aromatic”, “aromatic” and “anti-aromatic”. A concept map is a diagram that shows connections between concepts and should include as many details as you can.
-
Using your concept map, predict whether each of the following systems is aromatic, anti-aromatic or non-aromatic. Be sure to justify your choices.
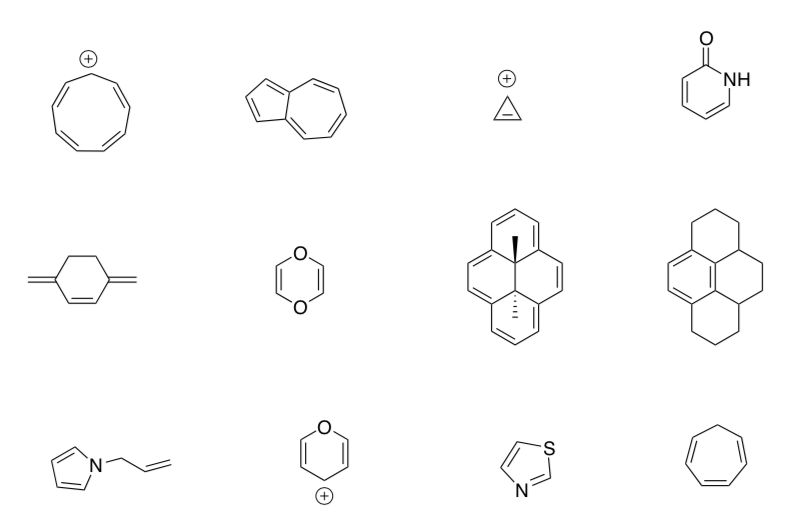
Application Problems:
-
Cyclooctatetraene
Experimental data suggests that cyclooctatetraene assumes a nonplanar, tub-shaped geometry. Each double bond is twisted relative to the adjacent double bonds.
-
Draw a Hückel MO diagram for cyclooctatetraene (use a Frost Circle). Include energy levels, electron filling and p labels. Label the HOMO and the LUMO.
-
Based on the MO diagram, would you predict this compound to be:
aromatic, anti-aromatic or nonaromatic
-
-
What is the hybridization of the carbons in this molecule?
-
What would you predict the bond angles to be?
-
Why does this compound bend to avoid planarity?
-
Based on this data, what would you predict for the bond lengths for this compound? Choose one option and explain:
Alternating single and double bonds:
C-C 1.5 Å
C=C 1.3 Å
or
Conjugated bonds (like benzene) 1.4 Å
-
Based on the experimentally determined geometry and bond lengths of cyclooctatetrene, would you predict this compound to be:
aromatic, anti-aromatic or non-aromatic
-
Draw the atomic orbital filling diagram for potassium metal.
-
Why does K metal readily donate electrons to become K+?
-
Cyclooctatetraene reacts with potassium metal to form the dianion. Draw the Hückel MO diagram of the dianion.
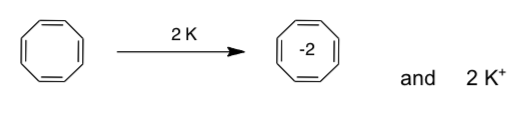
-
Based on the MO diagram of the dianion of cyclooctatetrene, explain why it is formed so readily.
-
-
Strength of Aromaticity
Aihara, Kanno, and Ishida, J. Phys. Chem. A 2007, 111, 8873-8876.
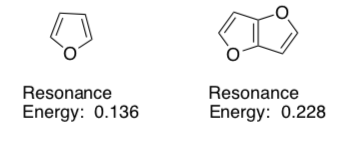
Within the framework of Hückel molecular orbital theory, aromatic resonance energy is an indication of the stabilization energy due to cyclic conjugation.
The authors of this paper calculated the resonance energy for a variety of compounds.
-
For these two aromatic compounds, explain the difference in resonance energy. Use resonance structures.
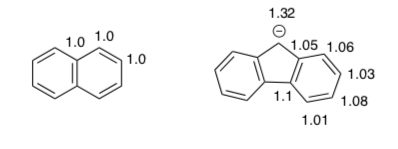
The authors of this paper also calculated the electron density on each atom for several structures.
-
For a very stable aromatic compound, what would you predict for the electron density on the atoms? Circle one.
All the same Alternating values High variation
-
For these two aromatic compounds, explain the difference in electron densities.
-
-
Aromaticity and Ferrocene
In 1950, R.D. Brown at the University of Melbourne predicted that the unknown molecule fulvalene would be nonaromatic.
Peter Pausen at Duquesne University decided to try to synthesize the molecule. The proposed synthesis involved this following first step:
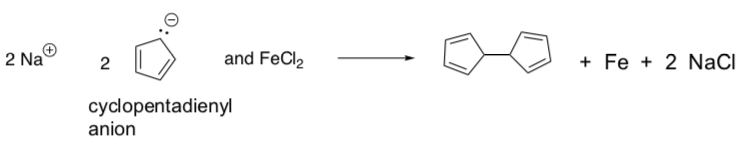
After completing the synthesis above, the data on the orange solid product suggested that one iron atom had become incorporated into the product.
The orange solid was found to have a melting point of 174 C and was soluble in benzene (C6H6) and ether (CH3CH2OCH2CH3).
-
What does the solubility and melting point data tell you about the compound? Is it likely to be a molecular structure or an ionic structure? Explain.
Pausen and his students proposed that the cyclopentadienyl anions had simply formed a coordination complex with the iron cation.
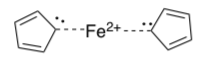
Is the cyclopentadienyl anion (circle one):
Aromatic Anti-aromatic Non-aromatic
-
-
Aromaticity and Ferrocene (cont.)
Nuclear Magnetic Resonance (NMR) spectrometry is a tool that can establish the number of different hydrogens in a molecule.
-
How many different hydrogen atoms are there in this structure?
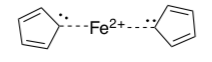
-
The H NMR of the reaction product was run and is shown below. (The peak at 0 on the x-axis may be ignored since it is due to a reference compound.) Can the structure above be correct?
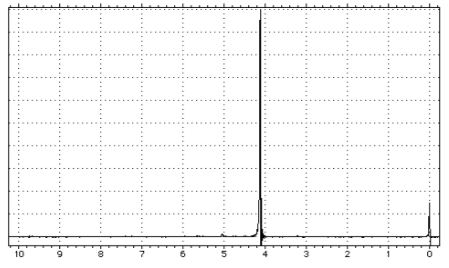
Eventually William von Eggers Doering suggested (but did not publish) the structure shown below.
-
Explain how this is consistent with the NMR spectrum. (Hint-the rings are parallel to each other with the iron in the middle.)
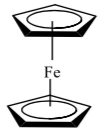
In a note submitted to the Journal of the American Chemical Society published on April 20, 1952, Sir Geoffrey Wilkinson and others proposed the now famous “sandwich” structure for the compound. In 1973, Wilkinson and E.O. Fischer received the Nobel Prize in chemistry for their work on the chemistry of organometallic sandwich compounds.
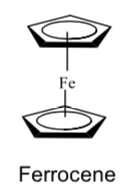
-
In ferrocene, the iron is formally in the +2 oxidation state. What is the valence electron configuration for Fe+2?
-
Assume that the ring is held to the iron by the interaction of pi MO’s on the cyclopentadienyl ring. How many p electrons can each Cp ring donate to the metal?
-
These compounds are often called pi complexes. Why?
-
What is the total electron count around the metal?
Wilkinson later said that workers at Union Carbide in the 1930’s noticed large amounts of an orange compound in iron pipes during the refining of petroleum but discarded it without characterizing it.
-
What was the structure of this compound?
-
Predict the structure of [Cr(benzene)2]. What about [Cr(CO)6]? [Cr(benzene)(CO)3]?
-

