12: Stereochemistry
- Page ID
- 142119
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Introduction to Chirality
Use this page to take notes while you complete the assignment in Sapling.
-
Draw structures in the boxes.
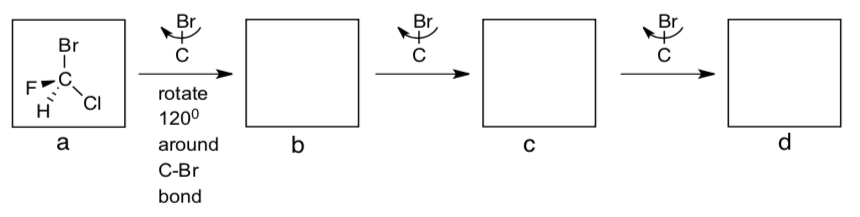
-
What is the relationship between a, b, c & d? Are they the same molecule?

-
What is the relationship between e, f, g & h?
-
What is their relationship with a, b, c & d?

-
What is the relationship between u, v & w?

-
What is the relationship between x, y & z?
-
What is their relationship with u, v & w?
Chirality
Chiral compounds that have non-superimposable mirror images are known asenantiomers.
Objects that are identical to their mirror image (i.e. superimposable with their mirror image) are not chiral. These objects (or molecules) are described as achiral.
-
Complete the following table.

Chiral Centers
A chiral center (stereocenter) is an atom bearing groups such that an interchanging of any two groups leads to a stereoisomer.
Note
P and S can both be chiral centers as the lone pair will count as one of the substituents but a neutral nitrogen atom is not a chiral center due to rapid inversion.
- A chiral center is a tetrahedral atom bonded to _________ different entities, such that an interchanging of any two groups gives rise to an enantiomer.
-
For the following compounds, circle each chiral center.
Hint: You may need to add in the missing hydrogen atoms.

Non-superimposable mirror images
While a chiral center is often the source of chirality, there are molecules that are chiral without a chiral center. There are also molecules that have chiral centers but the mirror image is superimposable upon it.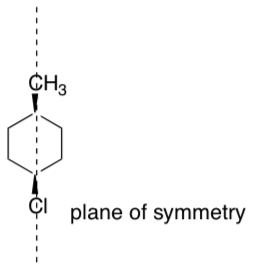
A better method for determining if a molecule will be chiral is to determine if a plane of symmetry exists. If a molecule has a plane of symmetry, it will be superimposable on its mirror image and is not chiral.
-
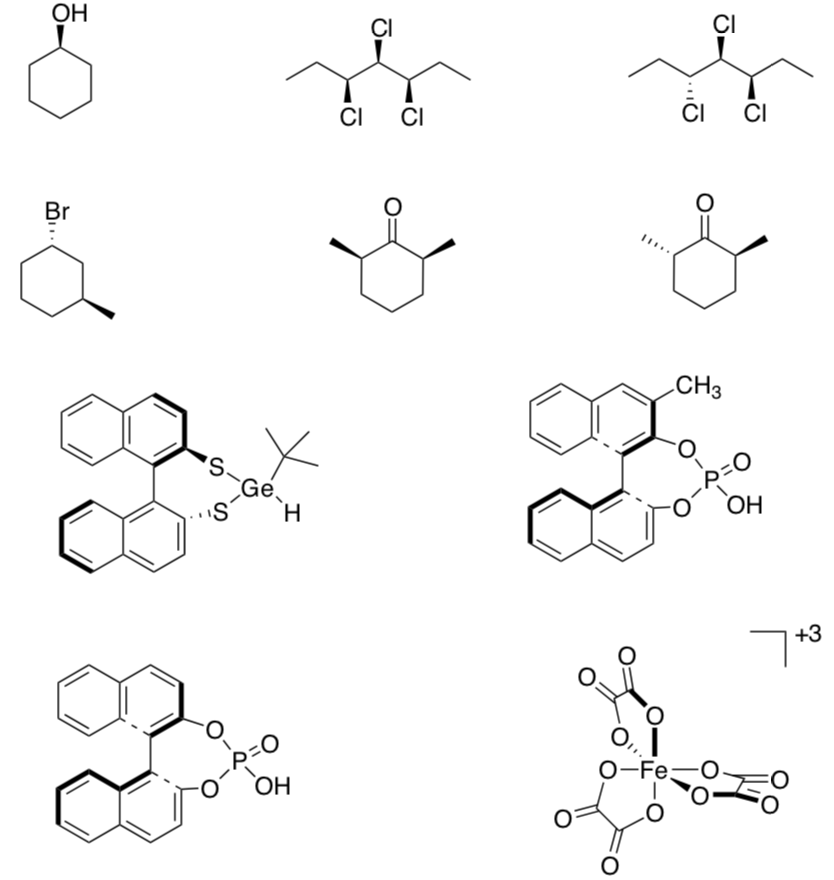
Draw a plane of symmetry through any symmetric Clmolecules.
-
Put a box around each of the chiral compounds.
-
Put a triangle around each of the achiral compounds.
Nomenclature of Stereochemical Centers: Cahn- Ingold-Prelog
I. Definitions and Introduction to Cahn-Ingold-Prelog Nomenclature for Stereocenters
Use this page to take notes while you complete the assignment in Sapling.
-
List the step-by-step rules for determining if a center is R or S.
-
More Practice: Label each of the chiral centers in these molecules with R/S designation.

Stereoisomers in molecules with multiple chiral centers
If there are two possible orientations for each chiral center, how many stereoisomers are possible for a molecule with
-
One chiral center?
-
Two chiral centers?
-
Three chiral centers?
-
What is the pattern? Is there a mathematical formula?
Consider the following molecule, 2,3-dihydroxybutanoic acid:

-
Circle the chiral centers.
-
How many possible stereoisomers?
-
Draw all possible stereoisomers. Hint: keep the carbons in a zigzag in the plane of the paper and use wedges & dashes to the OH groups. Use molecular models to build them if that helps.
-
Label each center with R/S.
-
Identify which pairs are mirror images of each other, enantiomers.
Diastereomers (diastereoisomers) are stereoisomers that are not enantiomers. Diastereomerism occurs when two or more stereoisomers of a compound have different configurations at one or more (but not all) of the equivalent (related) stereocenters andare not mirror images of each other.
-
Identify which pairs of 2,3-dihydroxybutanoic acid are diastereomers.
-
Indicate the relationship between each of the stereoisomers:
R, R S, S
R, S S, R
Stereoisomers in compounds with symmetry
Consider the following molecule, 2,3-butanediol:
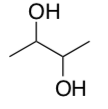
-
Circle the chiral centers.
-
Draw all possible stereoisomers. Use molecular models to build them if that helps.
-
Label each center with R/S.
-
Identify which pairs are mirror images of each other, enantiomers.
-
Are any of the mirror images superimposable? Remember, if mirror images are superimposable, the compounds are the same.
-
How many stereoisomers for this molecule?
-
Identify which pairs are diastereomers.
A meso compound or meso isomer is a non-optically active member of a set of stereoisomers, at least two of which are optically active. This means that despite containing two or more stereocenters (chiral centers) it is not chiral. A meso compound is superimposable on its mirror image, and it does not produce a "(+)" or "(-)" reading when analyzed with a polarimeter.
-
When will compounds have meso isomers?
-
Indicate the relationship between each of the stereoisomers below:
R, R S, S
meso
How to Determine Stereomeric Relationships

So You Think You Have Chiral Stereoisomers.

Identify the stereomeric relationships
-
Label each chiral center with R/S.
-
Determine whether the compounds in each of the following pairs arenonisomeric, identical, constitutional isomers, enantiomers, ordiastereomers.
-
Identify the chiral compounds.

Properties of Chiral Compounds
Enantiomers have the same physical properties (melting point, polarity, etc) except for:
- How they interact with polarized light.
- How they interact without other chiral compounds
1. Optical Activity
Because one enantiomer will rotate plane polarize light, chiral compounds are also called optically active compounds.

-
If a pure solution of one enantiomer rotates the light +36°, what would the optical rotation of the mirror image be?
-
A racemic mixture is a 50:50 mixture of two enantiomers. Will a racemic mixture be optically active? Explain your answer.
-
Would a pair of diastereomers have the same optical rotation?
-
What would you predict for the optical rotation of a meso compound?
2. Interaction with other chiral compounds
-
Are your feet chiral? Draw two feet. Are they superimposable?
-
Are your socks chiral? Does it matter which sock goes on which foot?
-
Are your shoes chiral? What happens if you put your shoes on the wrong feet?
Draw a cartoon to show the problem.
-
Explain why it matters which enantiomer of a medication you take. Consider how they might interact with chiral proteins/enzymes differently. Draw a cartoon.
-
Suggest a reason why two enantiomers might have very different flavors (eg R- carvone = spearmint, S-carvone = caraway).
Summary
-
Define the following terms:
- constitutional isomers
- enantiomers
- diastereomers
- optically active
- chiral compoundso chiral center
- achiral
- meso
- racemic mixture
-
List out the Cahn-Prelog-Ingold Nomenclature Rules for designating R/S.
-
What physical properties would be the different for enantiomers?
-
What physical properties would be the different for diastereomers?
More Practice
- R/S Practice
-
Identify the functional groups.
-
Circle the chiral center.
-
Label each chiral center with R or S.

-
-
Stereomeric Relationships
-
Determine whether the compounds in each of the following pairs arenonisomeric, identical, constitutional isomers, enantiomers, ordiastereomers.
-
Identify the optically active (chiral) compounds.
-
Identify any meso compounds.

-

