3.4: Solubility
- Page ID
- 137012
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Name: ______________________________
Section: _____________________________
Student ID#:__________________________
Template:HideTOCWork in groups on these problems. You should try to answer the questions without referring to your textbook. If you get stuck, try asking another group for help.
Prelude
One of the other general properties of ionic compounds is that they often have some solubility in water. The oceans, of course, are saltwater. In a mixture, two or more materials are mixed together but they remain essentially separate, like sand and water. You can still easily tell the difference between the sand and the water, because even if you shake them up they will separate again on their own.
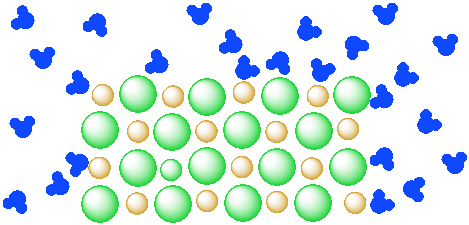
Figure \(\PageIndex{1}\): A mixture of an insoluble salt (orange and green ions) and water (blue molecules). The two components remain separate from each other.
In a suspension, one or more materials is mixed into a liquid, and the mixture becomes somewhat homogeneous. Instead of having easily identifiable layers, the liquid looks the same throughout. However, suspensions are generally cloudy liquids. Milk is a suspension. It contains water, fats and proteins. They may settle out into separate layers eventually, but it takes time. In a solution, one or more materials is mixed into a liquid, and the mixture becomes a completely homogeneous liquid. Solutions are transparent, not cloudy. They may be colored or colorless, but you can always see through them. Saltwater is a solution.
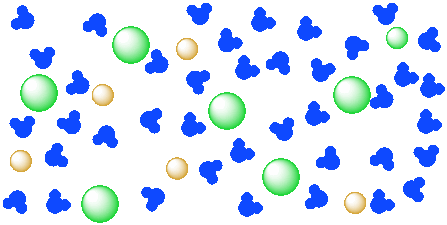
Figure \(\PageIndex{2}\): A solution of a salt (orange and green ions) in water (blue molecules). The ions of the salt are completely distributed throughout the water.
You cannot see chunks of salt in the solution because the salt particles are too small for you to see. The salt is separated into individual ions, surrounded by water molecules. Of course, if you put some salt in water, it might not dissolve right away; you might have to stir it for a while.
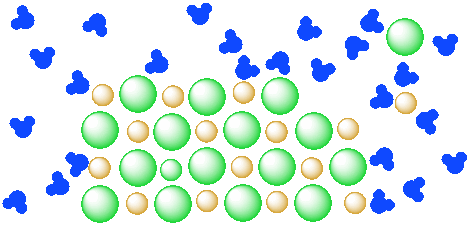
Figure \(\PageIndex{3}\): A mixture of a salt (orange and green ions) and water (blue molecules). The salt is beginning to dissolve in the water.
Eventually more of the salt would dissolve in the water.
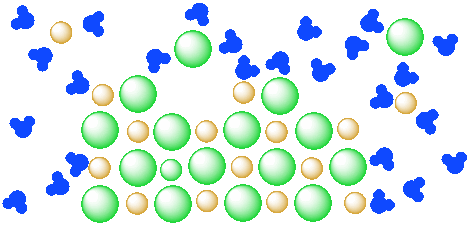
Figure \(\PageIndex{4}\): A mixture of a salt (orange and green ions) and water (blue molecules). The salt continues to dissolve in the water.
However, at some point, the system might come to "equilibrium": the water has dissolved all of the salt that it can, so the rest of the salt stays solid. This equilibrium may be "dynamic": different ions may become dissolved in the water or may be deposited from solution into the solid state. However, the overall ratio of dissolved ions to water stays the same.
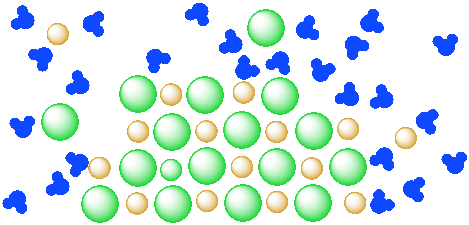
Figure \(\PageIndex{5}\): A mixture of a salt (orange and green ions) and water (blue molecules). The salt is partly dissolved in the water but has reached equilibrium.
Problem \(\PageIndex{1}\)
Let's take a look at the idea that a given amount of water is only able to dissolve a specific amount of salt.
- In the diagram above, how many water molecules are there?
- How many units of salt (an anion and a cation) are dissolved?
- If there were only a dozen water molecules present, how many units of salt would dissolve?
- If a hundred water molecules were present, how many units of salt would dissolve?
Why do salts dissolve in water? Water is a molecular compound; the atoms are directly attached to each other, rather than being ions that are attracted to each other. Because of electronegativity differences, the oxygen in water has a partial negative charge and the hydrogens have partial positive charges. Ionic compounds can dissolve in polar liquids like water because the ions are attracted to either the positive or negative part of the molecule.
Note that there is a sort of tug-of-war involved when things dissolve in water. The water is pulling individual ions away from the solid. The solid is pulling individual ions back out of the water. There exists an equilibrium at some point, based on how strongly the water attracts the ions, versus how strong the ionic solid attracts the ions.
You might expect to be able to predict vaying degrees of solubility in water for different ionic compounds. You would just use the principles of Coulomb's law that we used in melting points. The smaller the ions, the closer together they would be, and the harder it would be for the water molecules to pull the ions away from each other.
Problem \(\PageIndex{2}\)
Predict which of the following pairs should be more soluble in water, based on what you know about Coulombic attraction between ions.
- LiF or NaF
- NaK or KF
- BeO or LiF
Problem \(\PageIndex{3}\)
Although lithium fluoride and magnesium oxide contain cations and anions of roughly the same size, lithium fluoride is much more soluble in water (2.7 g/L) than magnesium oxide (0.087 g/L) at room temperature. Propose a reason why.
However, the trends we saw in melting points in ionic compounds become more complicated when it comes to solubility. The water solubility of alkali chlorides does not follow a simple trend (Table \(\PageIndex{1}\)).
| Compound | Water Solubility in g/L at 20oC |
|---|---|
| LiCl | 83 |
| NaCl | 359 |
| KCl | 344 |
Lithium chloride is certainly the least water-soluble of the three compounds. That makes sense, since the lithium ions are small and the attraction for the chloride would be stronger over that shorter distance. However, we would expect potassium chloride to be the most soluble by far, and it is hardly different from sodium chloride.
Problem \(\PageIndex{4}\)
Propose an explanation for why the water solubility of the alkali chlorides does not simply increase as the cation gets larger. If we change the halides, we see similar trends.
| Compound | Water Solubility in g/L at 0oC |
|---|---|
| LiCl | 83 |
| LiBr | 166 |
| LiI | 150 |
Once again, it is not surprising that the lithium chloride is the least soluble, but the most soluble seems to be the lithium bromide, not the lithium iodide.
This sort of behavior, in which we start to see a trend but it then reverses, often means there is more than one factor at work. In this case, there are a couple of other factors, some of which are more complicated. One of them simply involves the fact that there are two interactions going on here. We are not just overcoming the attraction of the ionic solid for individual ions, like when something melted. In this case, there is also the attraction of the water for the ion to think about. That attraction should also be governed by Coulomb's Law. At some stage, there must be a tipping point, when the factors that increase attraction between the ions also increase the attraction between the ion and the water. One or the other of these factors may become the dominant player under different circumstances.
- Several interactions are involved in dissolution.
- Cation - anion attraction is just one of these interactions.
- Cation - water and anion - water interactions are important, too.
- Water - water interactions also play a role.
Problem \(\PageIndex{5}\)
Let's review some basic points about ionic solids.
- Define lattice energy.
- What two properties affect the lattice energy of an ionic compound?
- Does a stronger or weaker lattice energy result in a stronger ionic bond?
- How will a strong lattice energy affect melting and boiling points of a crystal lattice?
- How will a strong lattice energy affect solubility of a crystal lattice?
Problem \(\PageIndex{6}\)
In each pair, determine which compound will have a higher lattice energy.
- NaCl or NaBr
- KF or CaF2
- MgO or Na2O
- KF or CsCl
- RbBr or CaCl2


