Literature Article Analysis: Electrochemistry (Pompano)
- Page ID
- 283249
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Article:
A. C. Schmidt, X. Wang, Y. Zhu, and L. A. Sombers, “Carbon Nanotube Yarn Electrodes for Enhanced Detection of Neurotransmitter Dynamics in Live Brain Tissue,” ACS Nano, 2013, 7(9), 7864-7873.
Learning Objectives:
After completing this paper day on FSCV, a student will be able to:
- Describe the role electrode size can play in cyclic voltammetry.
- Draw a cyclic voltammetry waveform and the corresponding voltammagram.
- Describe the key differences between fast scan and regular cyclic voltammetry, and the impact this can have on the observed voltammagrams.
- Interpret cyclic voltammetry data and use it quantitatively to analyze concentrations.
- Discuss the differences between microelectrodes types and report on a recent advancement made in electrode materials, CNT yarn.
- Discuss some examples of how microelectrodes are used in bioanalytical applications, based on the paper at hand.
Name:__________________________________
Microelectrode in vivo Electrochemistry
Article:
A. C. Schmidt, X. Wang, Y. Zhu, and L. A. Sombers, “Carbon Nanotube Yarn Electrodes for Enhanced Detection of Neurotransmitter Dynamics in Live Brain Tissue,” ACS Nano, 2013, 7(9), 7864-7873.
Pre-Class Questions About the Paper
- Summarize the purpose of the study in one complete sentence.
- What are the advantages of using microelectrodes compared to larger electrodes? What are the specific advantages of carbon-fiber microelectrodes?
- What properties of CNTs make them so special? What was the authors’ rationale in spinning carbon nanotubes (CNTs) into a yarn to make an electrode?
- One of the applications discussed in detail in the paper is the electrochemical detection of dopamine. Look up and draw the half-cell reaction for the oxidation of dopamine to dopamine-o-quinone.
- The authors compare their CNT-yarn electrode to standard cylindrical carbon-fiber microelectrodes in terms of quantitative analysis of neurotransmitters. Look at Figure 5 and the accompanying text, and figure out how the calibration curve in Figure 5b was constructed. Specify what the standard solutions were, the setup of the electrochemical cell, and describe how the values plotted on the y-axis were obtained.
In-Class Questions
- Why would it be important to use small electrodes to make measurements in living systems?
2A. For standard CV, a typical scan rate is 100 mV/s to 1 V/s. What scan rate was used in most of these FSCV experiments? Is this high or low compared to standard CV?
2B. In FSCV, why might non-faradaic currents be especially a problem? How do the authors mitigate this issue?
2C. How many cyclic voltammograms (scans) were acquired per second? Why is going fast important for measurements in the brain?
- Sketch the potential applied to the working electrode as a function of time. Include as much detail in your plot as possible (i.e. label everything, including specific times and voltages).
- Consider the dopamine CVs done with carbon-fiber electrodes in Figure 2b of the ACS Nano paper (reprinted below). Pay attention to which way the potential is plotted on the x-axis! Label the oxidation and reduction half-waves. Was the oxidation of dopamine reversible? How can you tell?
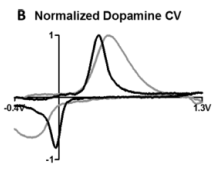
- Looking at Figures 5C-D, describe two ways that increasing scan rate affects the measurement.
- Looking at the data in the paper, compare the sensitivity (slope of the calibration curve) and LOD of quantification of dopamine using carbon fiber and nanotube yarn electrodes. Which method is more sensitive? Which has a lower LOD? Why might that be?
- Could CNTy electrodes be used to detect dopamine, hydrogen peroxide, adenosine, serotonin, ascorbic acid, and DOPAC all at once in a living brain sample, as a multiplexed measurement? What do you base your conclusion on?
- Suggest some advantages and disadvantages of electrochemical detection of dopamine in the brain, compared to spectroscopic detection.
Contributors and Attributions
- Rebecca Pompano, University of Virginia (rrp2z@virginia.edu)
- Adapted from an activity developed by Jill Venton, University of Virginia
- Sourced from the Analytical Sciences Digital Library


