Gas Chromatographic Columns
- Page ID
- 284024
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Learning Objectives
All students are expected to:
- Differentiate different types of columns (i.e., WCOT, PLOT, packed), their dimensions, applicability and diffusion factors affecting the separation
- Evaluate suitability of common stationary phases for different analytes
- Distinguish types of interactions between the analytes and stationary phase
- Justify the selection of a stationary phase based on its interactions, stability, and bleed with the targeted analyte
- Evaluate and explain the column bleed
Quiz GC Columns
Provide handwritten answers and bring them to the class
- Compare capillary and packed columns, their dimensions, the common number of theoretical plates, and factors affecting peak broadening.
- Explain the principle and strength of dispersive interactions (London forces), dipole-dipole interactions, hydrogen bonding.
- Differentiate stationary phases and discuss their relationship to the interactions with analytes.
- What is the difference in the interactions with analytes on methyl and phenyl dimethyl polysiloxane stationary phases?
- A homological series of alkanes is analyzed using a linear temperature gradient. What defines the elution order? How does Δtr change for sequentially eluting compounds?
- What are PLOT columns and how are they used?
- What is the column bleed?
- In GC analysis, what is the source of an ion of 207 m/z?
A. Stationary Phases and Integrations in GC
- Match a suitable stationary phase to the following analytes
Heptane C7H16

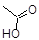
Heptanoic Acid C7H14O2
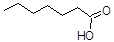
Glycerol C3H8O3
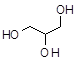
Nitrobenzene C6H5NO2
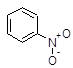
Acetic Acid C2H4O2
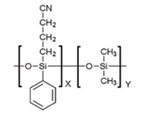
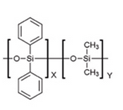
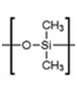
- List the types of interactions expected between the analytes and stationary phases selected above.
- Which of the stationary phases above would be suitable specifically for GC-MS analysis?
- Which of these stationary phases is the least thermally stable?
B. Separation on PLOT columns
- What are PLOT columns? Please sketch.
- Which of the analytes listed below would be separated on a PLOT column?
- Organic solvents
- Polycyclic aromatic hydrocarbons
- Refinery gases
- Air
- Fatty acids
- Which analytes (of those listed above) would not be suitable for a PLOT column and why?
- Sketch two chromatograms of air on an alumina PLOT column with retention H2< O2 < N2, CO2 using a column with stationary phases of 0.1 µm and 5.0 µm film thickness.
- Would you expect to have longer or shorter retention times on a thicker stationary phase and why?
- How fast would be the elution of the analytes listed above?
- Based on the points 5 and 6 reconsider your chromatograms.
- Would it better work with higher or lower flowrates (Consider Van Deemter theory)
C. GC Analysis and Column Bleed
- Sketch a chromatogram of a solvent (hexane) analyzed using the temperature program from 40 °C to 320 °C with a 25 °C/min gradient.
- What is the column bleed? Consider whether it would affect the sketched chromatogram above?
- Sketch three chromatograms and label all peaks of a series of alkanes (C7-C15) dissolved in n-hexane on a dimethyl polysiloxane stationary phase analyzed using a split injection and
- An Isothermal analysis at 200 °C.
- A temperature program from 40 °C to 320 °C with a 5 °C/min gradient.
- A temperature program from 100 °C to 320 °C with a 5 °C/min gradient.
- Do you expect any co-elution? Why?
D. Optimizing Temperature Program
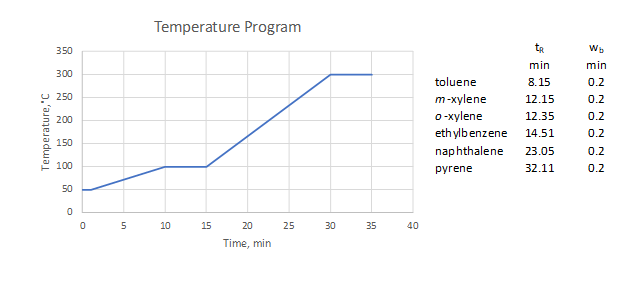
- The temperature program and analytes elution profile are shown above. How could you modify the temperature program to improve the analysis?
- Determine temperatures at which each analyte eluted in the temperature program used above.
- Is your program shorter or longer than the original?
- Based on questions 2 and 3, reconsider the proposed program and suggest a new one.
Contributors and Attributions
- Alena Kubatova, University of North Dakota (alena.kubatova@und.edu)
- Sourced from the Analytical Sciences Digital Library


