Absorbance and Fluorescence Analysis of NAD and NADH
- Page ID
- 293926
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Case Study
Multi-modal spectrophotometric analysis of NAD and NADH
Learning Goals:
- Consider the effect of electronic structure on Abs and Fluor Em.
- Design experiments using Abs and Fluorescence.
- Compare calibration curves using Abs and Fluorescence.

Do you know what your redox state is? [1]
Because the enzyme cofactors NAD and NADH differ in their interaction with light, it is possible to quantitate enzymatic reactions based on light absorbance or by fluorescent emission. In this activity, you will consider why this difference occurs, and how it is used to our advantage for analytical chemistry.
Part I.
Why do NAD and NADH have such different spectra?[2]
- To begin to answer this question, first identify the difference in structure between NAD and NADH. Circle the relevant area of the molecule in the figure above, and draw any relevant electrons.
- We learned previously that absorbance and emission arise from transitions between molecular orbitals. Let’s briefly consider benzene and pyridine.
- What distinguishes pyridine from benzene? Draw the relevant electrons into the picture.
- Benzene has a set of conjugated p-bonds, and the highest occupied molecular orbital (HOMO) is shown in the electronic diagram below. What is the lowest energy transition?
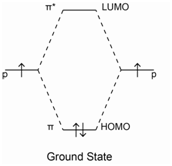

- Look up the UV-Vis spectrum of benzene in the NIST Webbook and sketch it in the box above. Label the axes and identify the λmax. Is this transition detectable on a typical UV-Vis?
- Electrons in lone pairs are referred to as non-bonding electrons, and their energy is similar to what it would be in the atom alone. Below, add the non-bonding electrons (labelled “n”) to the energy diagram we had for benzene. What is the lowest energy transition now? Do you expect the corresponding absorption to have a longer or shorter wavelength?


- Look up the UV-Vis spectrum of pyridine in the NIST Webbook and sketch it in the box above. Label the axes and identify the λmax. Is this transition detectable on a typical UV-Vis?
- What distinguishes pyridine from benzene? Draw the relevant electrons into the picture.
- Returning to system at hand, briefly explain whether you expect NADH or NAD to be more likely to absorb in UV-Vis range, and why.
Part II.
Spectrometric assays and instrumentation
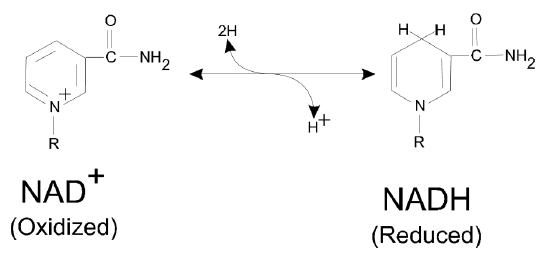
NAD is a soluble dinucleotide that can be reversibly reduced by the addition of 2 hydrogen ions to form NADH. Because of their different optical properties, we can quantify reactions that convert one to the other. A common example is the reaction of an enzyme called lactate dehydrogenase (LDH) with NAD:
\[\mathrm{Lactate + NAD \xrightarrow{\substack{\textrm{Lactate } \\ \textrm{dehydrogenase}}} Pyruvate + NADH}\nonumber\]
- You want to design an assay to quantify the conversion of NAD to NADH after a 60-sec reaction with the enzyme LDH. But, you don’t know the absorbance max of NADH, and cannot find it in the NIST webbook. What could you do in the lab to solve this problem?
- How should the instrument be configured to perform this experiment? Sketch the basic instrumental setup. What part of the instrument has to move to collect the data you need?
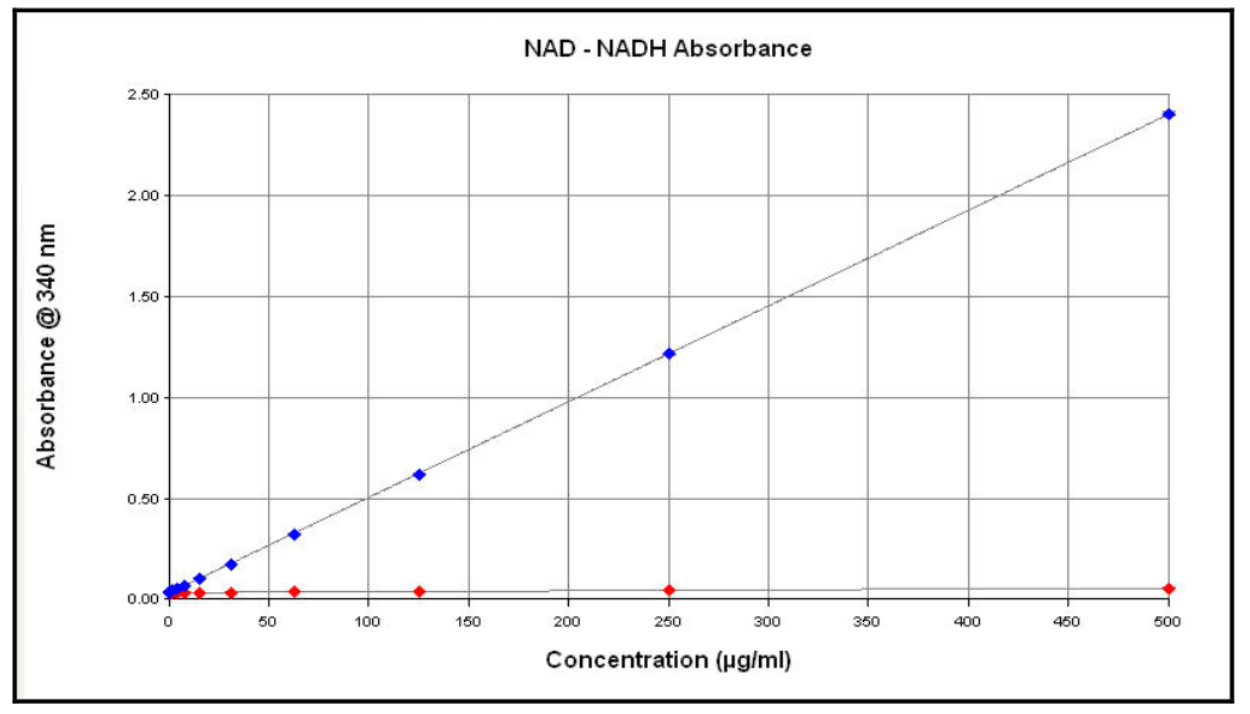
- The absorbance spectrum for NAD and NADH is shown below.1
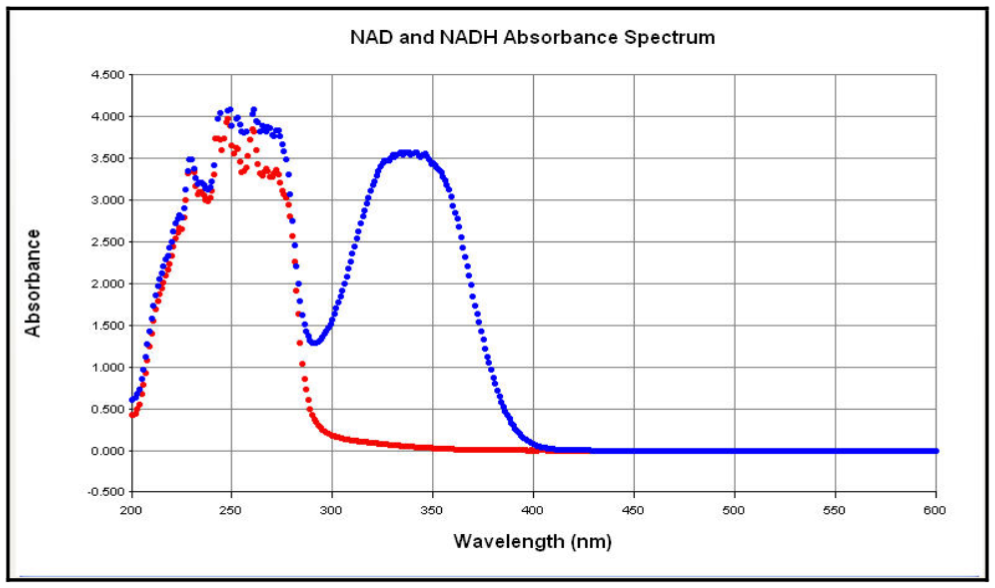
Figure 3. Spectral Scans of NADH and NAD+ solutions. Aliquots (100 µl) of NADH and NAD solutions (1 mg/ml) were aliquoted into half area-UV transparent plates and a spectral scan from 200 nm to 600 nm in 1 nm increments performed. Label which curve is which on the graph, based on your considerations in part I.
What wavelength would be most useful to detect, in order to monitor the conversion of NAD to NADH by UV-Vis spectroscopy?
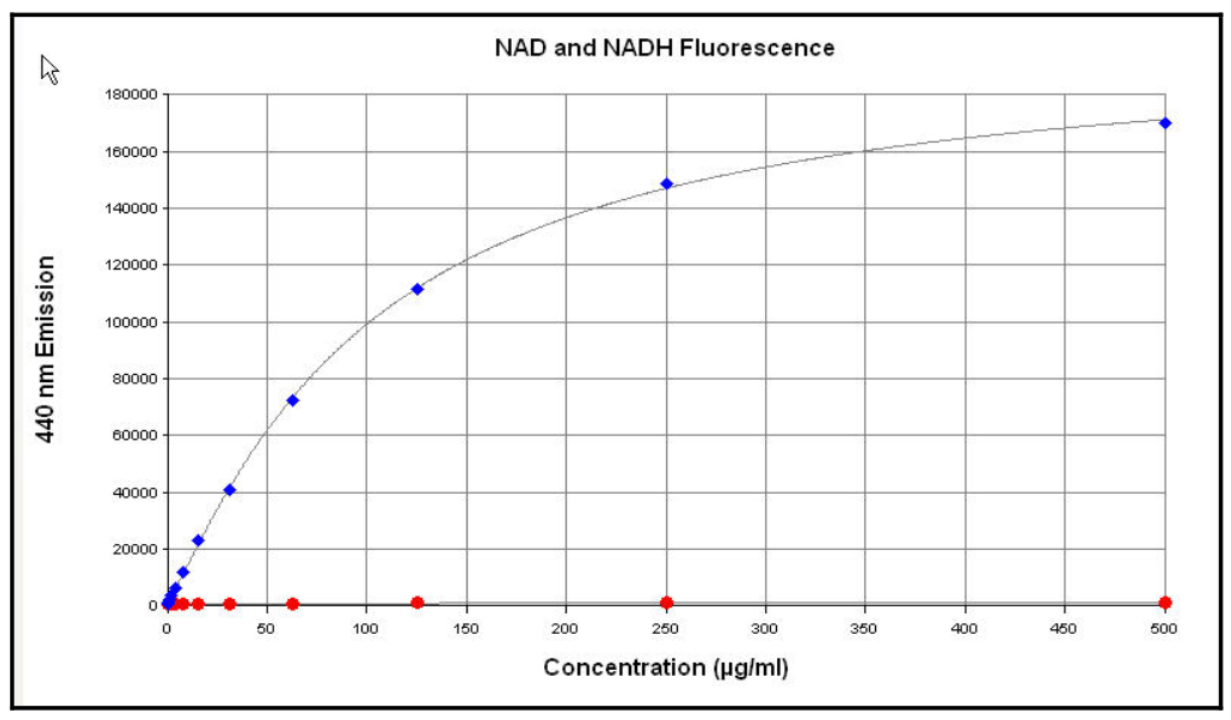
- Remembering back to your homework, you know that in addition to absorbing, NADH also fluoresces (emission max 465 nm). NAD does not fluorescence at this wavelength. How should the instrument be configured to measure fluorescence at this wavelength? Be specific about wavelength selection.
- You prepare a set of standard solutions of NADH, ranging from 0 to 500 µg/ml, in a simple buffer called TE buffer.
- Do you expect to obtain better sensitivity (lower LOD) by measuring absorbance or fluorescence? Why?
- Do you expect to obtain a higher upper limit of the linear range by measuring absorbance or fluorescence? Why?
- Do you expect to obtain better sensitivity (lower LOD) by measuring absorbance or fluorescence? Why?
- Look at the data in Figures 4 and 5. Do they confirm your prediction from question 8b?
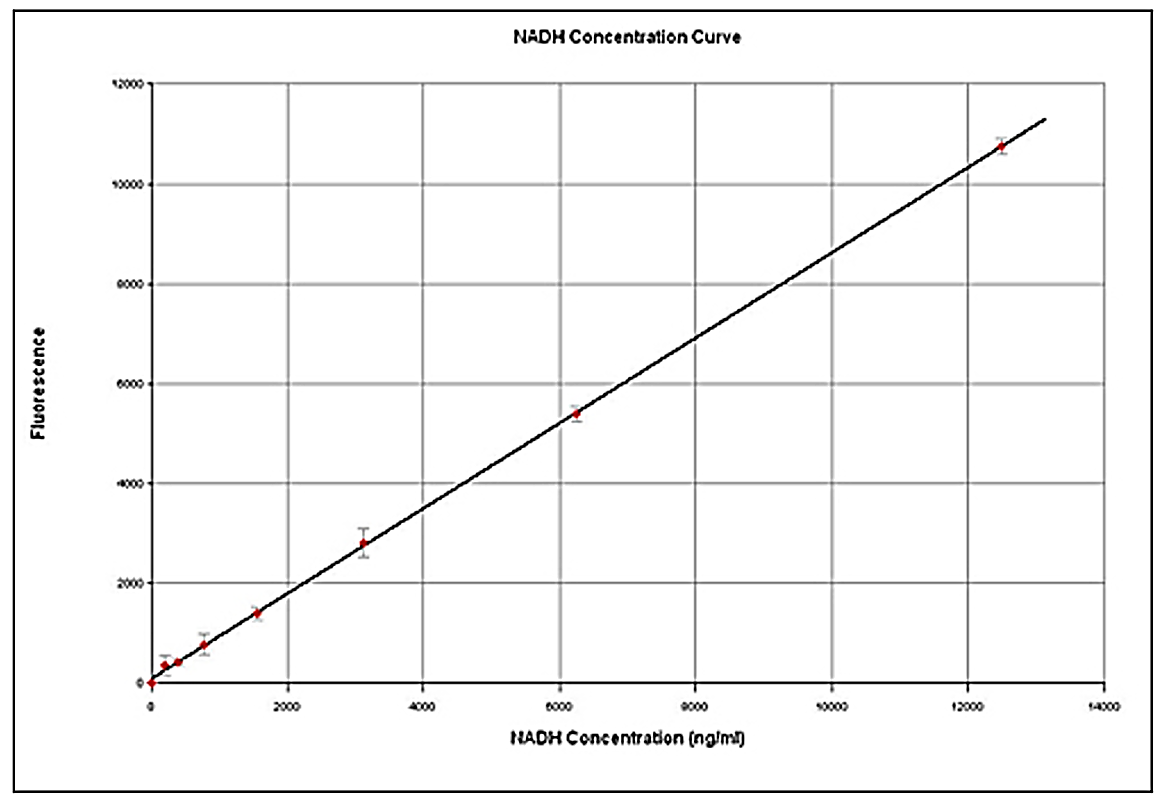
- What could you do to get a linear standard curve for fluorescence of NADH?
- Now compare Figures 4 and 6. Do they confirm your prediction from question 8a? How much better is one method than the other?
- What type of fluorescence detector would be more useful for detection of very low concentrations of NADH, a photodiode or a PMT? Why?
References
[1] Adapted from: Paul Held, “Determination of NADH Concentrations with the Synergy™ 2 Multi-Detection Microplate Reader using Fluorescence or Absorbance,” BioTek Application Note, 2006. Accessed Sept. 14, 2018. https://www.biotek.com/resources/application-notes/determining-nadh-concentrations-with-synergy-2-multi-mode-microplate-reader-using-fluorescence-or-absorbance/
[2] Adapted from: Tom Wenzel, ASDL Spectroscopy Unit, Bates College.
Contributors and Attributions
- Rebecca Pompano, University of Virginia (rrp2z@virgina.edu)
- Sourced from the Analytical Sciences Digital Library


