7: Carbon NMR
- Page ID
- 332807
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)13C NMR Spectroscopy
Nuclear magnetic resonance (NMR) spectroscopy involves the molecules absorbing electromagnetic energy in the radiofrequency range. Spin (on either an electron or a proton) has two possible values: up and down. NMR excitation involves the excitation of nuclei to a different possible spin state when placed in a magnetic field.
Imagine a sample with lots of nuclei. Ordinarily, nuclei with up and down spins have the same energy, so the spins are random.

Spins in a Magnetic Field
In an NMR experiment, the sample is placed in a magnetic field. In the external field, the spins tend to either align with the magnetic field or line up against the magnetic field. In a magnetic field, the two spin states have slightly different energies. A narrow majority of electrons will adopt the lower energy state.
- Add several spins to the diagram below.

Radio-frequency radiation is sent into the sample, and a few of the nuclei are promoted to the next level.
- Fill in the spins after irradiation:

NMR Experiment
NMR measures wavelength of energy emitted as the nuclei ‘relax’ back to the ground state (aligned with the magnet).
- Fill in the spins after relaxation.

The frequency that a nucleus absorbs is mostly dependent on the bonding environment of the atom (chemical shift).
- This chemical shift value tells us about types of bonding.
- The number of different frequencies absorbed tells about the number of unique nuclei.
13C NMR Spectroscopy Analysis
Two types of information are found in the 13C – NMR spectra:
- Number of carbons: Each unique carbon atom appears as a signal/peak.
- Chemical Shift (\(\delta\)): The location of each peak depends on the chemical environment of the carbon atom.
Let's look at both of these factors:
- Number of Unique Carbons
- Make a model of pentane.

- Hold it up to and have a teammate point to a specific carbon. Spins it a few times in the air and ask them to show you the carbon they originally selected.
- You will notice that the molecule has symmetry and there are some carbons that are identical.
- How many sets of equivalent carbons are there in pentane?
- Only unique carbons will show up in the 13C NMR spectrum. So, there will be ____________ peaks for pentane.
Symmetry and Peaks in NMR
Only unique carbons will show up in the 13C NMR spectrum. So, there will only be three peaks for pentane (previous page).
- Predict the number of peaks that would be present in the 13C spectrum for each of these compounds. Indicate symmetry.

- Aromatic compounds are often seen in the laboratory. Predict the number of peaks that would be present in the 13C spectrum for each of these compounds. Remember that these compounds are flat. Indicate symmetry.

Chemical Shift (bonding environment)
There are two factors that affect chemical shift:
- Geometry
- Dipole
Geometry
sp3 is tetrahedral (carbon with 4 single bonds)
sp2 is trigonal planar (carbon with two single bonds and one double bond)

Dipole
As the carbon of a functional group becomes more partially positive, it will appear further downfield (larger ppm). The carbon will feel more positive if it is bonded to a more electronegative atom.
- Order the following functional groups by the dipole strength. (1 = carbon that feels the most neutral; 5 = carbon that feels the most positive.) Use the periodic table to evaluate the electronegativity of atoms.

Analyze 13C NMR Spectra with Shift and Symmetry*
* All spectra are either from SDBS (Japan National Institute of Advanced Industrial Science and Technology) or simulated.
Alkanes
- How many unique carbons in 2-methylbutane? Show a plane of symmetry.

- Are the carbons sp3 or sp2? Where would you expect to see the peaks?
This is the spectrum of 2-methylbutane.

- Typically, sp3 C that are in non-polar bonding environments appear below _______ ppm.
Alcohols and Amines
1-pentanol and 1-pentyl amine are shown below.
Sp3 carbons attached to a more electronegative atom are pulled downfield (to the left).
- sp3 C-O are often between 50-80 ppm
- sp3 C-N are often between 40-60 ppm
- sp3 C-Cl are often between 35-80 ppm
- sp3 C-Br are often between 25-55 ppm
For these examples, determine
- How many unique carbons?
- Which is the atom bonded to the oxygen or nitrogen?


Alkenes:
Alkenes have sp2 carbons which typically appear between 120-160 ppm.
This is a 13C NMR spectrum of 1-hexene: 
- Identify the two sp2 carbons in the structure above.
- Identify the two sp2 carbons in the spectrum below.

Aromatic Rings:
This is the spectrum of toluene (methylbenzene)
For this structure, determine
- How many unique carbons? Show the structure with a plane of symmetry.
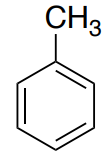
- Where will the sp3 CH3 appear?
- The aromatic ring C atoms will appear between 120-160. Identify these atoms.

Aromatic Ring with a Heteroatom
This is the spectrum of anisole (methoxybenzene).
- How many unique carbons? Show the structure with a plane of symmetry.
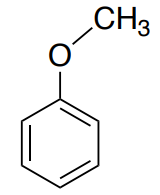
- Where will the sp3 CH3 appear?
- Identify the peaks representing the atoms of the aromatic ring.
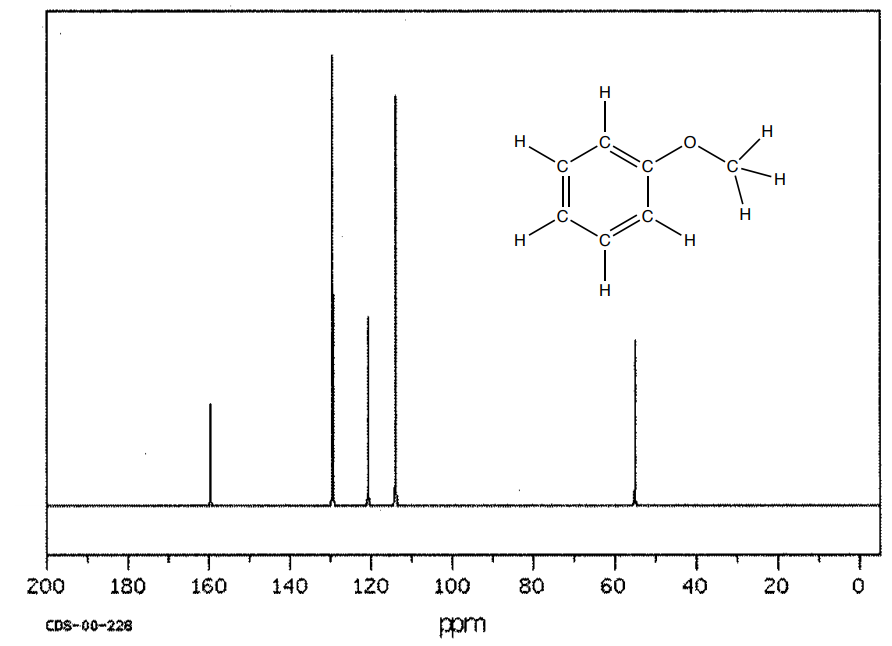
- How does the shifts of the methyl and the ring change with the presence of the oxygen? (Compare to spectrum of toluene on previous page).
Carbonyl Compounds: Ketones and Aldehydes
This is the spectrum of 2-pentanone. 
- How many unique carbons? Show the structure with a plane of symmetry.
- Where will the sp3 carbons appear?
- Where does the C=O appear?
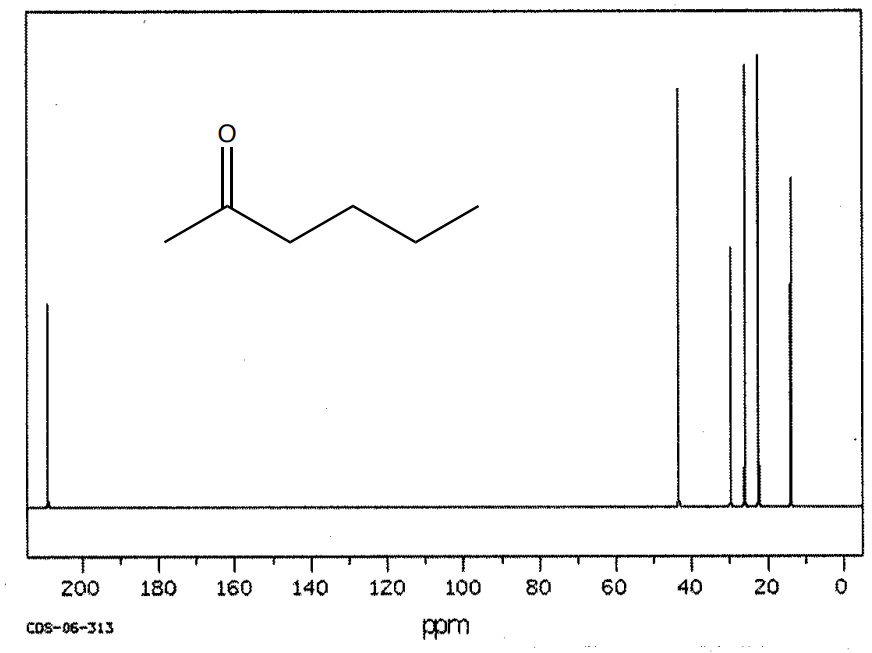
Carbonyl carbons (aldehydes and ketones) appear between 200 and 220 ppm
Carbonyl Compounds: Acid Derivatives
The carbonyl group of acid derivatives such as esters and amides are shifted relative to the C=O of aldehydes and ketones.
- Assign the peaks for the structure of propyl acetate below.

- Comment on the shift of aldehyde/ketones vs esters/amides.
Summary of Chemical Shift Regions

Note: The carbon atom in CDCl3 (typical solvent) appears at 77 ppm
IR and 13C Application Problems
- Complete data tables for the spectra.
- Propose possible structures for the following sets of IR and 13C NMR spectra.
A. C3H8O

| Frequency | Functional Group |
|---|---|
| 3350 | |
| 2950 | |
| 1450 | |
| 1150 | |
| 950 |

| Chemical Shift | Type of Bonding Environment | Proposed Structure: |
|---|---|---|
| 10 | ||
| 25 | ||
| 62 |
B. C3H8O

| Frequency | Functional Group |
|---|---|
| 3400 | |
| 2990 | |
| 1450 | |
| 1125, 1175 | |
| 9750 |

| Chemical Shift | Type of Bonding Environment | Proposed Structure: |
|---|---|---|
| 22 | ||
| 62 |
C. C3H6O

| Frequency | Functional Group |
|---|---|
| 3000 | |
| 1720 | |
| 1450 |
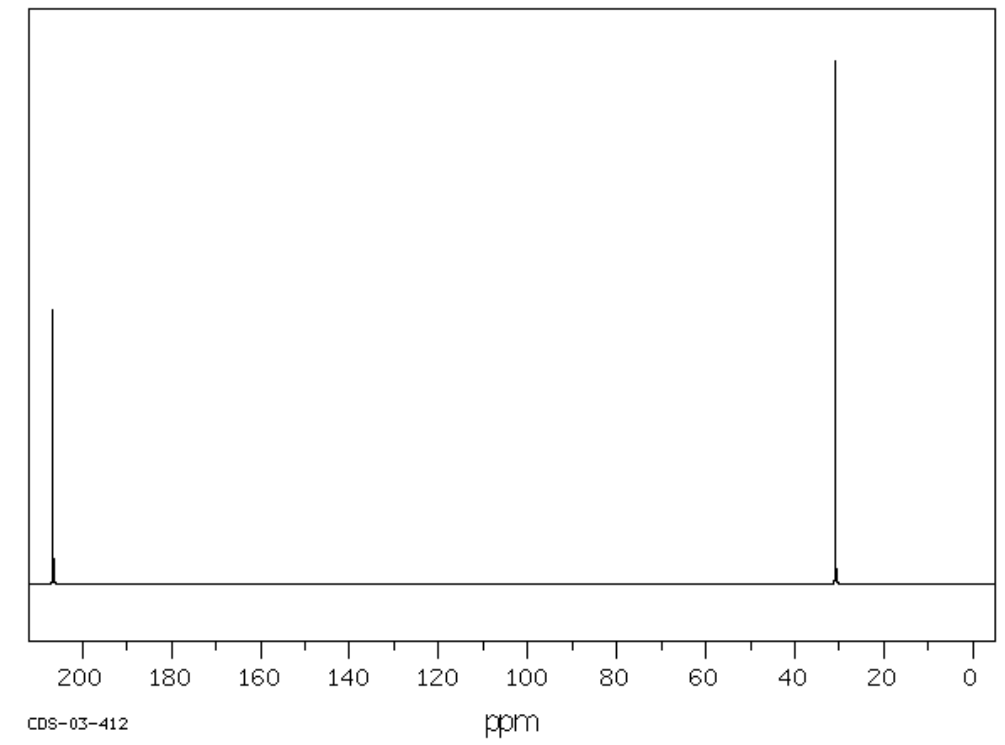
| Chemical Shift | Type of Bonding Environment | Proposed Structure: |
|---|---|---|
| 30 | ||
| 205 |
D. C3H6O


| Frequency | Functional Group |
|---|---|
| Chemical Shift | Type of Bonding Environment | Proposed Structure: |
|---|---|---|
E. C5H10O

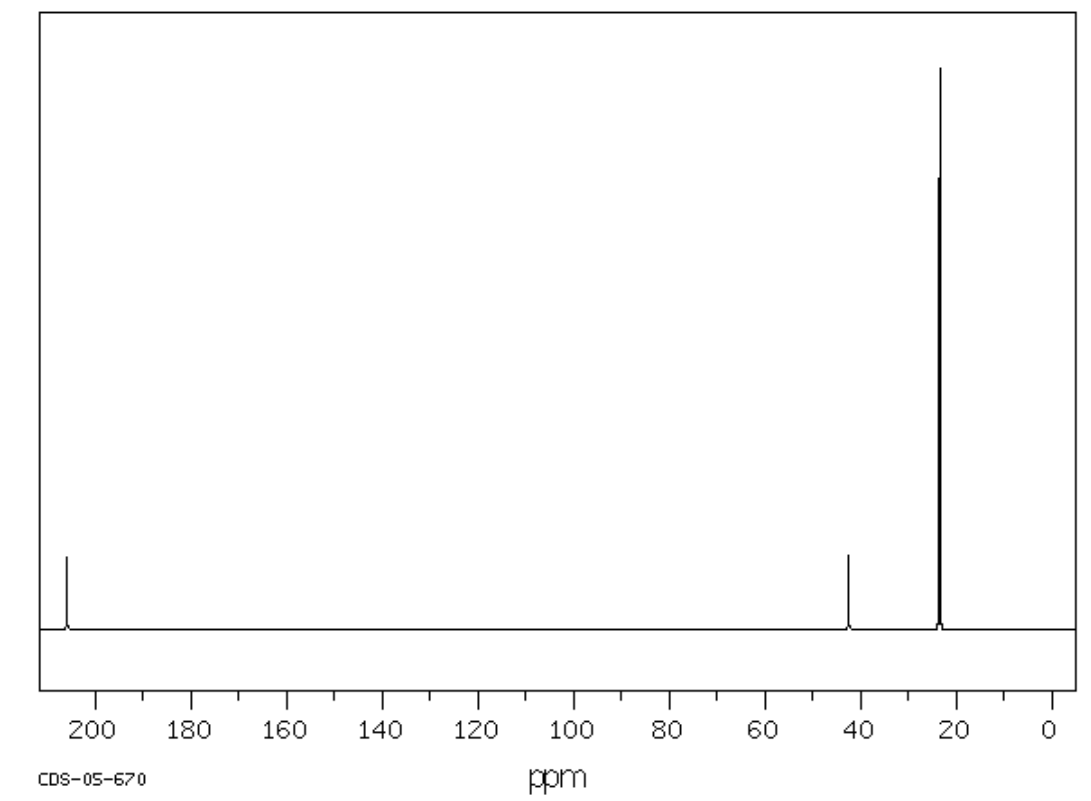
| Frequency | Functional Group |
|---|---|
| Chemical Shift | Type of Bonding Environment | Proposed Structure: |
|---|---|---|
F. C3H6O


| Frequency | Functional Group |
|---|---|
| Chemical Shift | Type of Bonding Environment | Proposed Structure: |
|---|---|---|
G. C8H8O (note: there are two peaks at about 130 ppm in the 13C-NMR)


| Frequency | Functional Group |
|---|---|
| Chemical Shift | Type of Bonding Environment | Proposed Structure: |
|---|---|---|
H. C8H8O2 (note: there are four peaks between 122 and 135 ppm in the 13C-NMR)


| Frequency | Functional Group |
|---|---|
| Chemical Shift | Type of Bonding Environment | Proposed Structure: |
|---|---|---|
I. C6H10O


| Frequency | Functional Group |
|---|---|
| Chemical Shift | Type of Bonding Environment | Proposed Structure: |
|---|---|---|
J. C5H10O2 (there may be more than one possible structure for this)

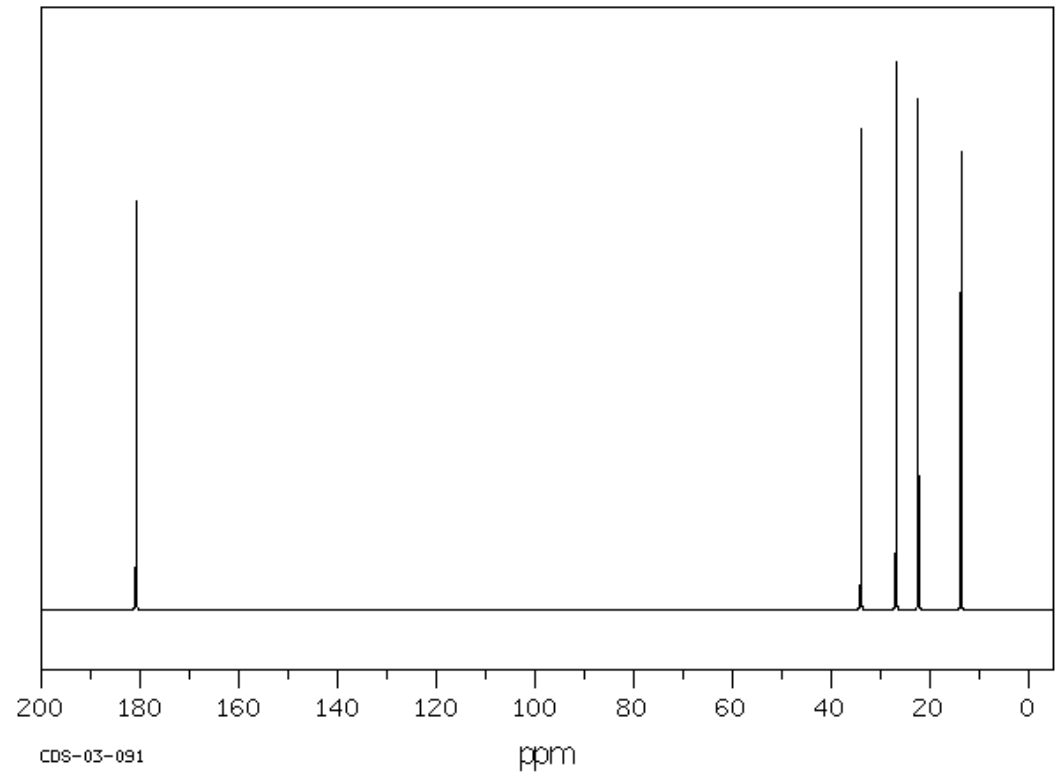
| Frequency | Functional Group |
|---|---|
| Chemical Shift | Type of Bonding Environment | Proposed Structure: |
|---|---|---|
K. C6H14O (there may be more than one possible structure for this)


| Frequency | Functional Group |
|---|---|
| Chemical Shift | Type of Bonding Environment | Proposed Structure: |
|---|---|---|
L. C6H12O2 (there may be more than one possible structure for this)


| Frequency | Functional Group |
|---|---|
| Chemical Shift | Type of Bonding Environment | Proposed Structure: |
|---|---|---|
M. C8H9NO (there may be more than one possible structure for this)


| Frequency | Functional Group |
|---|---|
| Chemical Shift | Type of Bonding Environment | Proposed Structure: |
|---|---|---|


