23.2: The Chemical Shift
- Page ID
- 214098
1. The nucleus under observation is typically a part of a whole molecule. This means that the electron density around different nuclei is not the same. For example “acidic protons” (those bonded to highly electronegative atoms) have less electron density than protons bonded to carbon. Hydrogen bonded to metals (also known as hydride) carries a partial negative charge and therefore has a high degree of electron density:

2. The electrons that surround the nucleus are also magnetically active and they generate their own tiny magnetic vectors. In the presence of an external field these electronic vectors oppose the nuclear vector. Therefore the electrons act as tiny shields, protecting the nucleus from the full effect of the external field. The higher the electron density, the more “shielded” the nucleus is from the external field. For example, if we could place HCl and NaH under the same magnetic field, the hydrogen nuclei in these compounds would not experience the field strength to the same extent. The hydrogen in NaH would be more shielded than the hydrogen in HCl due to its higher electron density.
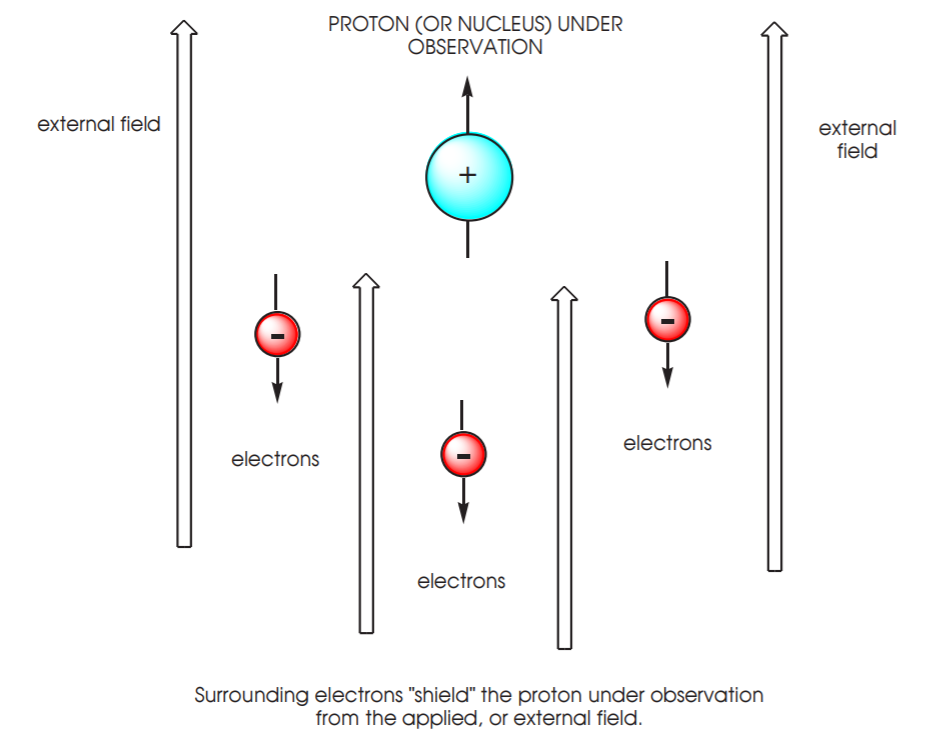
3. Hydrogen, or proton, is the most extensively studied nucleus due to its high natural abundance (100%) and widespread presence in organic molecules. In a given molecule different types of protons are surrounded by different degrees of electron density, and therefore experience different degrees of electron “shielding” in an NMR experiment.
4. Protons which are in areas of high electron density, such as the hydrogen atom in NaH, are more highly shielded. They will experience a weakened external magnetic field relative to protons which are in areas of low electron density (and therefore less shielded) such as the hydrogen atom in HCl. As a result, the proton in NaH will require energy of lower frequency to achieve resonance than the proton in HCl, which is exposed to the full strength of the external magnetic field.
5. The difference in resonance frequency between chemically different types of protons forms the basis for their differentiation and is called the chemical shift. The chemical shift scale has experienced several changes over the years. Most changes are designed to standarize the results of NMR experiments performed with different magnets under different conditions. In this way, a given molecule will turn the same chemical shift values regardless of whether the experiment was performed on different instruments or under different conditions.
6. The currently used chemical shift scale is called the δ scale. In this scale the x-axis represents the chemical shift of the proton under observation relative to a standard called tetramethylsilane, or TMS. The chemical shift value is reported in parts per million (ppm). The proton absorption for TMS is always assigned an arbitrary value of zero ppm. All other protons in the spectrum are then referenced to the TMS peak. The δ-scale increases from right to left and has a typical range of about 10-12 ppm for most common organic compounds. The y-axis in the δ-scale represents the intensity of the absorption.
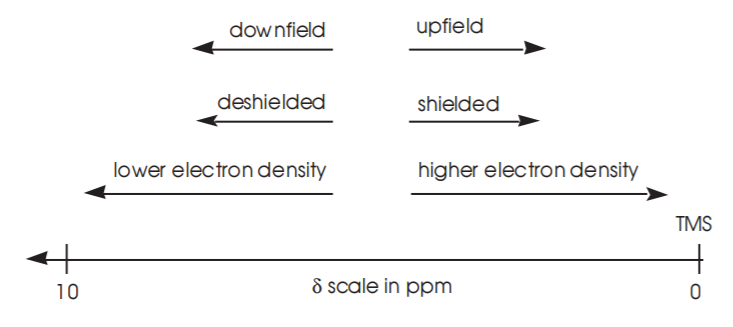
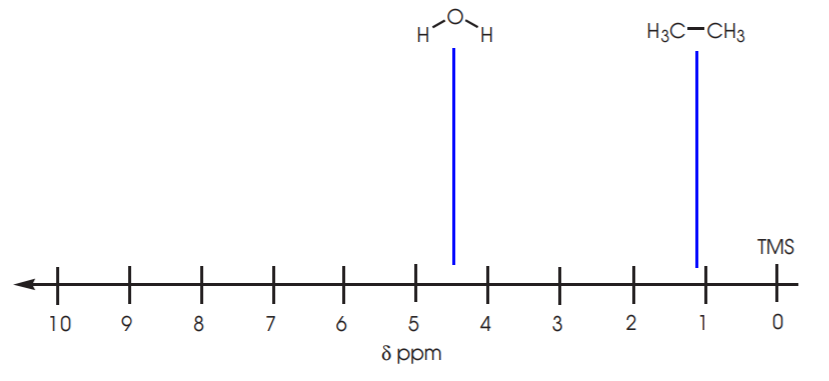
7. In the δ-scale more highly shielded protons appear at lower δ values, whereas relatively deshielded protons appear at higher δ values. Therefore protons attached to carbon, which is less electronegative than oxygen, will be more shielded and will typically appear around 1 ppm. Protons attached to oxygen, or those which are relatively close to more electronegative atoms, will be deshielded and will appear at higher δ values, as shown below for the . Refer to your textbook for more examples.
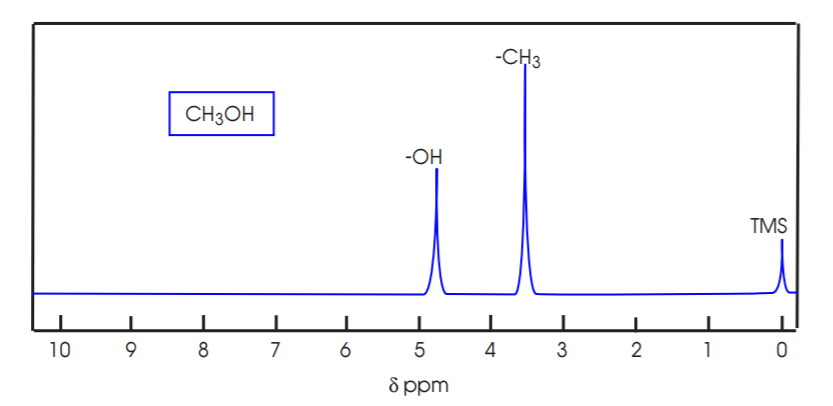
8. The NMR spectrum of methanol exemplifies this effect in a single molecule. The protons attached to carbon produce a signal located higher upfield (lower δ value) than the proton attached to oxygen.