13.1: The Aldol Condensation and Crossed Aldol Reactions
- Page ID
- 213819
The effect of the dipole moment of the carbonyl group on adjacent atoms is an inductive effect that results in lowered electron density in the surroundings. This effect makes the carbon atom electrophilic, and it also makes the adjacent hydrogens acidic. The acidic protons are capable of reacting with strong bases such as hydroxide ion to yield the conjugate base, a resonance stabilized enolate ion.
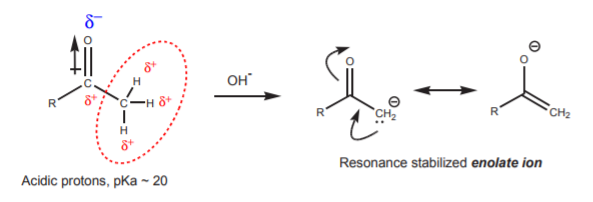
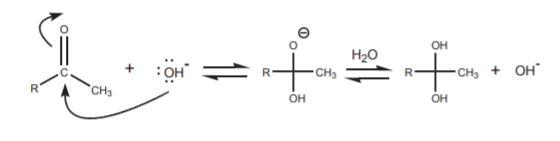
This is one of the several processes that can take place when the carbonyl compound is in the presence of base. Another process is, of course, a nucleophilic attack of the hydroxide ion on the carbonyl carbon. This process is synthetically inconsequential because all it does is to establish an equilibrium between the free carbonyl compound and its hydrate, two forms of the same functional group.
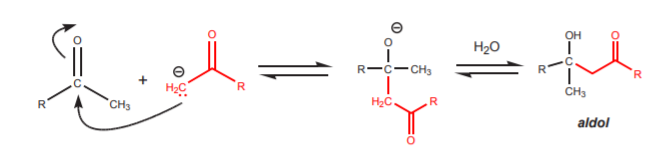
Even if only small amounts of enolate ion form, that is enough to trigger another reaction that can be synthetically consequential, and that is the aldol reaction. The enolate ion can act as a nucleophile and attack the carbonyl carbon of another molecule, leading to a dimer called aldol. The word aldol stands for aldehyde-alcohol, although it is frequently a misnomer, as when ketones are used instead of aldehydes.
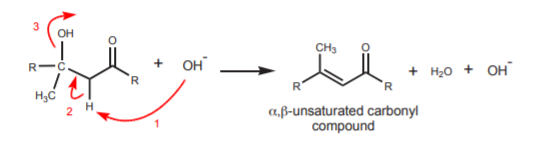
This reaction can take place at room temperature or at low temperature (~5o). If heat is applied, the aldol can dehydrate to form an alkenecarbonyl called an \(\alpha\) , \(\beta\) -unsaturated carbonyl compound. The protons \(\alpha\) to the carbonyl are still acidic and can be abstracted by base. This can lead to movement of electrons to form a new \(\pi\)-bond that is in conjugation with the carbonyl \(\pi\)-bond. This requires departure of the hydroxyl group. Even though this is not a good leaving group, the reaction can be driven towards formation of the \(\alpha\) ,\(\beta\) -unsaturated carbonyl compound by the higher temperature and by the thermodynamic stability of the conjugated product.
The synthetic usefulness of this reaction is obvious from the fact that it provides a tool for the coupling of two molecules which can be either the same or different. This process can be used to produce a variety of carbon structures that can be tailored according to need. \(\alpha\) , \(\beta\) -Unsaturated carbonyl compounds are important in the synthesis of many natural products and drugs. For example, collagen is the most abundant fibrous component of skin, bone, tendon, and teeth. One of its functions is to hold cells together in discrete units. Collagen fibers are strengthened by crosslinking of aldehyde functional groups through aldol condensations.
Aldol condensations performed with two different carbonyl compounds are called crossed aldol condensations. In order for them to be synthetically useful, one must have the required \(\alpha\)-protons, but the other should not. If both molecules have \(\alpha\) -protons then a mixture of products with similar structures will result which will have to be separated and the yields of the desired product will be lower.
In this experiment, a benzaldehyde derivative will be used as the carbonyl compound that contains no \(\alpha\)-protons, and acetophenone will act as the carbonyl compound containing \(\alpha\)-protons. The products are a series of analogs called benzalacetophenones, or chalcones.


