1.1: Purpose and Background
- Page ID
- 211975
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
The principal aims of this experiment are:
- To provide experience in the synthesis of a relatively simple covalent compound, bis(pentahaptocyclopentadienyl) iron, ( η 5-C5H5)2Fe, whose trivial name is ferrocene (1).
- To become familiar with inert atmosphere techniques.
- To introduce the use of thin-layer chromatography as an analytical tool and column-chromatography as a means of purification.
In the course of this synthetic sequence, the student will encounter operations such as sublimation, distillation, and execution of reaction under an inert atmosphere, all common techniques in synthetic chemistry.
Background:
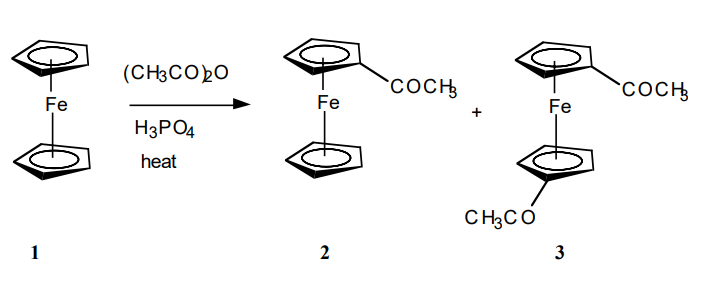
Ferrocene is a historically important molecule. The recognition of the "sandwich" structure of C10H10Fe in 1951-spawned transition metal based organometallic chemistry. This field is still developing and has produced a huge number of compounds in which saturated, unsaturated, and aromatic organic fragments are bonded directly to metal centers.
Ferrocene exhibits the properties of a typical aromatic molecule. The compound is stable to more than 500 °C. It does not react readily with acids or bases; however, it is sensitive to oxidizing agents. All the carbon atoms in the two-cyclopentadiene rings are bonded equally to the central ferrous ion by the π electrons of the two rings. Ferrocene does not undergo addition reactions typical of cyclopentadiene, but is readily subject to electrophilic aromatic substitution. Depending upon the catalyst (AlCl3, H3PO4) and the reaction conditions, either the monosubstituted product (2) or the disubstituted product (3) is the major product of acetylation. For a particular set of reaction conditions, the student will determine whether the major product is the orange acetylferrocene (2) or the red 1,1’-diacetylferrocene (3).