8.E: Exercises
- Page ID
- 438383
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Properties of Gases
Matching: Which pair correctly describes measurements of pressure, volume, amount, and temperature?
a. 2.20 atm
b. 305 mL
c. 234 \(^{\circ}\)F
d. 3.50 g
Which cylinder has the highest pressure?
a. Cylinder 2
b. Cylinder 3
c. Cylinder 1
d. Cylinder 1 and 3
e. Cylinder 2 and 3
Which of the following physical quantities does not describe characteristics of gases?
a. Pressure
b. Temperature
c. Concentration
d. Volume
e. Amount of Gas
Which of the following is not a typical unit of pressure?
a. atm
b. psi
c. mmHg
d. kPa
e. mol
Which of the following statements about gasses is incorrect?
a. Gasses are small particles that move randomly and have high velocities.
b. Gas particles are in constant motion and move in straight lines.
c. An increase in temperature will increase the kinetic energy of the particles.
d. Gasses expand to fill the volume of a container.
e. Gas particles are strongly attracted to each other.
Gas Pressure
A barometer is usually filled with ________ and used to measure ________.
a. water, volume
b. alcohol, temperature
c. liquid, moles
d. mercury, pressure
In a weather report, the atmospheric pressure is given as 19.4 inches of mercury. What is the corresponding pressure in mmHg? Note: 1 in = 2.54 cm and 1 cm = 10 mm.
a. 67.2 mmHg
b. 202 mmHg
c. 747 mmHg
d. 493 mmHg
e. 668085 mmHg
The pressure in gas cylinder 2 is 2.25 atm. What is the pressure in cylinder 1 and 3?
a. Cylinder 1 = 2.25 atm and cylinder 3 = 6.25 atm
b. Cylinder 1 = 6.25 atm and cylinder 3 = 4.50 atm
c. Cylinder 1 = 6.75 atm and cylinder 3 = 4.50 atm
d. Cylinder 1 = 2.25 atm and cylinder 3 = 6.78 atm
The partial pressure of \(\ce{O2}\) in alveolar air in the human lung is approximately 104 mmHg at sea level. However, for a climber at the summit of Mt. Everest, where atmospheric pressure is lower, the \(\ce{O2}\) pressure in the alveolar air of a climber is 43 mmHg, which reduces the distribution of oxygen to the muscles. What is the difference in these two values in units of atmospheres (atm)?
a. 0.80
b. 0.080
c. 61
d. 61.0
e. 12.5
Intraocular pressure (IOP) is the fluid pressure inside the eye. A person with an IOP greater than 22 mmHg may suffer from glaucoma or ocular hypertension. Convert this pressure to atmospheres and report your answer in 2 significant figures.
Gas Laws
What will happen to a bag of chip with an initial volume of 225 mL and seal level pressure of 760 mmHg when it is taken onboard an airplane that is pressurized to 635 mmHg? What is the new volume?
a. The bag will expand to a new volume of 269 mL
b. The bag will not change size and remain at a volume of 225 mL
c. The bag will shrink to a volume of 189 mL
d. The bag will double in size to a volume of 450 mL
What happens to the pressure when the gas sample is heated with volume constant?

a. The pressure will increases as described by Gay Lussac’s Law
b. The pressure will decreases as described by Gay Lussac’s Law
c. The pressure will stay the same as described by Gay Lussac’s Law
d. The pressure will increases as described by Boyle’s Law
What happens to the pressure of the gas sample when the volume is halved and its Kelvin Temperature double?

a. The pressure will double
b. The pressure will be halved
c. The pressure will stay the same
d. All of the above
(Multi select) There are four physical quantities (\(P, V, T, n\)) to describe properties of gases. Which gas laws reflect a variable relationship of two physical quantities and two other fixed physical quantities?
a. Ideal gas law
b. Boyle’s law
c. Dalton’s law
d. Charles’s law
e. Gay-Lussac’s law
Which gas law is a combination of multiple, simple gas laws?
a. Ideal gas law
b. Boyle’s law
c. Dalton’s law
d. Charles’s law
e. Gay-Lussac’s law
Which gas law is based on the sum of gas pressure contributions within gas mixture?
a. Ideal gas law
b. Boyle’s law
c. Dalton’s law
d. Charles’s law
e. Gay-Lussac’s law
A metal tank with a volume of 5.00 L is filled with nitrous oxide, an anesthetic used for surgery, to a pressure of 1.20 atm. If all of the nitrous oxide is transferred to a tank with a volume of 3.00 L, what is the new pressure in atm?
a. 12.5
b. 2.00
c. 1.00
d. 0.720
e. 7.20
f.
A gas has a volume of 4.00 L at 0.00 \(^{\circ}\)C. What final temperature, in \(^{\circ}\)C, is needed to change the volume of the gas to 1.50 L, if \(n\) and \(P\) do not change?
a. 455
b. -375
c. -102.
d. 728
e. -171
Identify the gas law described by the graph below:

a. Boyle’s Law
b. Charles’s Law
c. Avogadro’s Law
d. Gay-Lussac's Law
Based on the graph below, what would be the predicted volume of 6.50 mol of gas? (Hint, how can you use the line equation to solve this problem?).
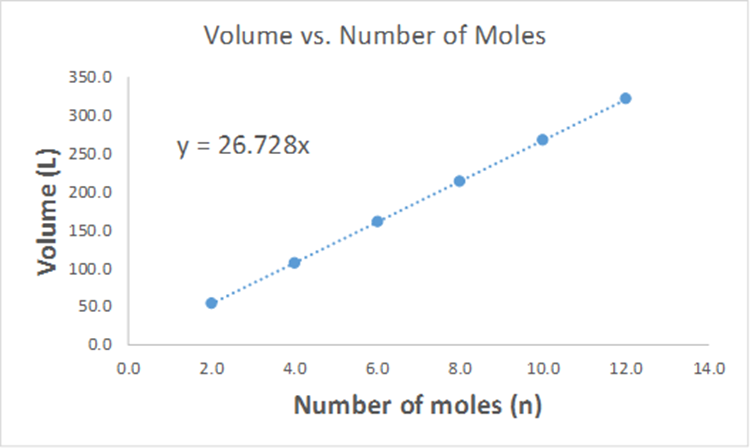
a. 4.00
b. 150.
c. 174
d. 77.0
e. 200.
A sample of helium gas has a volume of 6.0 L at 600. mmHg. What is the new pressure when the volume changes to 18.0 L at a constant \(T\) and \(n\)?
a. 1800 mmHg
b. 600 mmHg
c. 200 mmHg
d. 5 mm Hg
A sample of oxygen gas has a volume of 640. mL at a temperature of 23 \(^{\circ}\)C. At what temperature (in \(^{\circ}\)C) will the volume of the oxygen be 820. mL (\(P\) and \(n\) are constant)?
a. 106 \(^{\circ}\)C
b. 379 \(^{\circ}\)C
c. 231 \(^{\circ}\)C
d. 29 \(^{\circ}\)C
A sample of argon gas has a volume of 0.240 L, a pressure of 0.900 atm, and a temperature of 27 \(^{\circ}\)C. At what temperature (\(^{\circ}\)C) will the helium have a volume of 80.0 mL and a pressure of 3.20 atm (\(n\) remains constant)?
In problem solving, what is the acceptable temperature unit to substitute into the gas laws equations?
a. Fahrenheit
b. Celsius
c. Kelvin
d. All of the above
An air bubble happens to develop in an orifice within a deep sea diver when he is 63 ft below sea level. At this depth and assuming constant temperature, the bubble has a volume of 0.013 mL and a pressure of 3.53 atm. What is the volume of the bubble at the surface where the pressure is 1.00 atm?
An adult human lungs can only hold a volume of up to 6.0 L of air when fully expanded. Suppose that at sea level where the pressure is 1.00 atm, a sky diver traps 1.2 L of air in her lungs and holds her breath before rapidly ascending to a higher altitude where the pressure drops to 0.55 atm. If she doesn’t exhale, determine if her lungs will rupture at this high altitude, then complete the sentence: At the higher altitude, the gas volume will be________ and the lungs will (rupture/not rupture) __________.
An adult human lungs can hold a volume of up to 6.0 L of air when fully expanded. Suppose that at sea level where the pressure is 1.00 atm, a sky diver traps 3.0 L of air in her lungs and holds her breath before rapidly ascending to an altitude where the pressure drops to 0.46 atm. If she doesn’t exhale, determine if her lungs will rupture at this high altitude, then complete the sentence: At the higher altitude, the gas volume will be________ and the lungs will (rupture/not rupture) __________.
On a hot summer day Dylan’s mom gets him a fully inflated birthday balloon at a nearby City Party. A few minutes after stepping out of the store, the balloon exploded just before she got into her car. Why do you think the balloon exploded?
a. balloon expanded due to increased atmospheric pressure
b. balloon expanded due to increased amount of air in it
c. balloon shriveled due to excessive pressure
d. balloon expanded due to increased temperature
On a hot summer day Dylan’s mom gets him a fully inflated birthday balloon at a nearby City Party. A few minutes after stepping out of the store, the balloon exploded just before she got into her car. What advice would you provide if City Party has to fill another balloon for her that same hot day?
a. fill balloon with cold air
b. fill balloon just like before
c. fill balloon with slightly less air than before
d. fill balloon with slightly more air than before
During an unusually cold summer morning when the temperature is 21.5 \(^{\circ}\)C, you add air to your car tires to a pressure of 33.2 psi. The day warms up rapidly while you drive around town. You take the pressure reading at 2 p.m. and it reads 36.0 psi. Assuming that the tire volume doesn’t change, what is the temperature of the air in your tires at 2 p.m.? Report answer to 1 decimal place.
Match the gas laws, Boyle’s, Charles’s, and Gay-Lussac’s with the following graph
a. 
b. 
c. 
Combined Gas Law
At what temperature (give your answer in \(^{\circ}\)C) does 250.0 mL of argon gas at room temperature (25 \(^{\circ}\)C) and 1.50 atm occupy a volume of 450.0 mL at a pressure of 1.90 atm?
Molar Volume at STP
What is the volume (L) of
a. 2.5 moles of oxygen at STP? __________
b. 2.5 moles of nitrogen at STP? __________
How many moles of helium occupy 44.8 L at STP?
At STP, there are 22.4 L of argon gas and 22.4 L of xenon gas. Which one is heavier?
What volume will 0.780 moles of He occupy at STP?
Ideal Gas Law
Potassium nitrate decomposes to potassium nitrite and oxygen. What volume, in liters, of \(\ce{O2}\) can be produced from the decomposition of 50.0 g of \(\ce{KNO3}\) at 35.0 \(^{\circ}\)C and 1.19 atm?
\[2\ce{KNO3} (s) \rightarrow 2\ce{KNO2} (s) + \ce{O2} (g) \nonumber\]
a. 0.596
b. 5.25
c. 120
d. 10.5
e. 1.21
A single patient hyperbaric chamber has a volume of 640 L. At a temperature of 24 \(^{\circ}\)C, how many grams of oxygen are needed to give a pressure of 1.8 atm.
a. 1500 g
b. 1200 g
c. 1100 g
d. 640 g
Dinitrogen oxide is used in dentistry as an anesthetic called laughing gas. What is the pressure of 0.700 mole of \(\ce{N2O}\) gas at 22 \(^{\circ}\)C in a 5.00 L container?
a. 3.39 atm
b. 2.40 atm
c. 1.39 atm
d. 5.34 atm
A sample of gas has a mass of 0.433 g and its volume is 248 mL at a temperature of 28 \(^{\circ}\)C and a pressure of 745 mmHg. What is the molar mass and identity of this gas?
a. \(\ce{CO2}\)
b. \(\ce{NH3}\)
c. \(\ce{O2}\)
d. \(\ce{N2}\)
e. \(\ce{CO}\)
What is the volume, in liters, of 4.00 moles of methane gas, \(\ce{CH4}\), at 18 \(^{\circ}\)C and 1.40 atm of pressure?
a. 4.22
b. 8.36
c. 42.2
d. 68.3
e. 0.517
6.7 liters of an ideal gas is contained at 2.5 atm and 45 \(^{\circ}\)C. How many moles of this gas are present?
A lung holding a residual air volume of 1.50 L at 36 \(^{\circ}\)C has a pressure of 1.00 atm. How many moles of residual air is the lung holding? \(R\) = 0.0821 L.atm/mol.K.
A tire in your car is filled with air with an internal volume of 30.0 L. Determine the number of moles of air in the tire when the temperature outside is 20 \(^{\circ}\)C and the pressure inside the tire is 3.50 atm.
A sample of gas has a mass of 0.311 g. At 55 \(^{\circ}\)C its volume is 0.225 L and its pressure is 886 mmHg. Find its molar mass.
Dalton’s Law of Partial Pressure
A tank of compressed air containing nitrogen, oxygen, water vapor, and carbon dioxide, has a total pressure of 5.0 atm. If the partial pressures of nitrogen, oxygen, and water vapor, are 2970.0 mmHg, 800.0 mmHg, and 28.5 mmHg, respectively, what is the partial pressure of carbon dioxide in mmHg?
a. 1.50
b. 15.0
c. 150.
d. 155.
e. 160.
One-fourth of a gaseous mixture is Argon and 3/4 of the gas is Oxygen. The total pressure in the container is 8 atmospheres. What is the partial pressure of the Argon?
a. 1 atm
b. 2 atm
c. 4 atm
d. 6 atm
e. 8 atm
A gas tank containing an air mixture of helium, nitrogen and oxygen has a total pressure of 45.0 psi. Answer the following questions given that the partial pressures of helium and nitrogen in the mixture are 7.50 psi and 13.5 psi, respectively, and there are a total of 6.75 moles of gas mixture in the tank. Report all answers to 3 significant figures.
a. What is the partial pressure of oxygen? Answer = 24.0 psi
b. Calculate the moles of oxygen in the tank. Answer = 3.60 mol
c. Calculate the moles of nitrogen in the tank. Answer = 2.03 mol
d. Calculate the moles of helium in the tank. Answer = 1.13 mol
The air that we breathe is actually a mixture of mostly the following gases— 78% \(\ce{N2}\), 21% \(\ce{O2}\), and (<1.0%) \(\ce{CO2}\) and \(\ce{H2O}\) and constitute about 99% of the air mixture on a poor air quality day. That remaining, approximate 1% of the air is associated with trace amounts of air and particle pollutants that can affect air quality, especially on hot, summer days. Groups that can be affected by poor air quality include those who have asthma, heart disease, and chronic obstructive pulmonary disease (COPD). If the total air pressure is 1.0 atm, what would be the partial pressure (atm) of \(\ce{O2}\) on a poor air quality day?
Kinetic Theory of Gases

Each of these flasks contains the same number of molecules at the same temperature. In which container is the pressure highest?
Four different flasks contain equal amount of gas samples at 298 K. In which of the following samples will the gas molecules be moving the fastest?

The four curves represent the relative proportion of gas molecules and molecular speed for four different gasses. Rank these gases according to molecular weight (1-lowest and 4-highest).
Use the kinetic molecular theory to identify the sample that can be easily compressed.
a. 
b. 
c. 
Using the kinetic molecular theory, explain why the gas molecules cannot have dipole-dipole attraction in the ideal gas condition.
Using the kinetic molecular theory, explain why the tire pressure in your car decreases in winter.

