6: Lewis Structures and Molecular Shapes (Experiment)
- Page ID
- 93987
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Practice drawing Lewis Structures for various covalently bonded molecules and polyatomic ions.
- To use model kits to construct these molecules/ions in order to explore their structure and shapes.
- To practice predicting molecular shapes (using VSEPR theory) and molecular polarit
Background
Non-metal atoms bond covalently, resulting in the formation of either neutral molecules or polyatomic ions. A covalent bond is formed when non-metal atoms share their valence electrons, which they do in order to achieve filled valence orbitals like their nearest noble gas neighbor. This means that most bonded non-metal atoms will acquire a total of eight valence electrons via the sharing process – often referred to as the octet rule. A notable exception is hydrogen, which only needs to acquire two electrons to be like its nearest noble gas neighbor, helium.
Lewis Structures
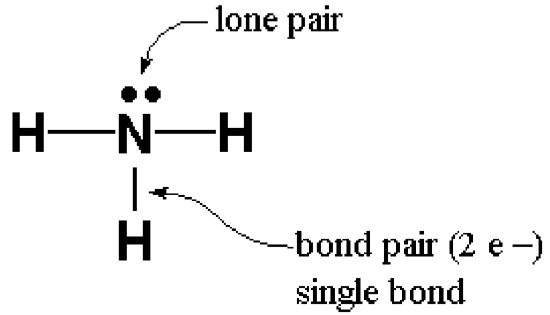
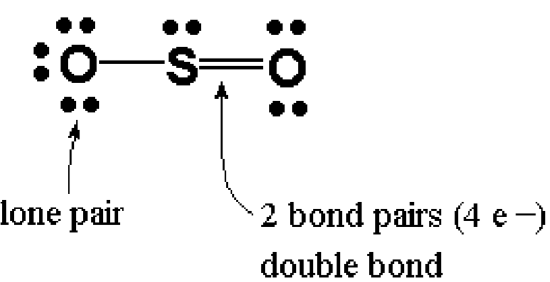
A Lewis Structure is a representation of covalent molecules (or polyatomic ions) where all the valence electrons are shown distributed about the bonded atoms as either shared electron pairs (bond pairs) or unshared electron pairs (lone pairs). A shared pair of electrons is represented as a short line (a single bond). Sometimes atoms can share two pairs of electrons, represented by two short lines (a double bond). Atoms can even share three pairs of electrons, represented by three short lines (a triple bond). Pairs of dots are used to represent lone pair electrons.
Examples:
The rules for drawing Lewis structures can be found in the Procedure Section of this handout.
Molecular Shapes
The shape of a molecule depends on the distribution of atoms in space about the central atom, and their bond angles. Bond pair electrons and lone pair electrons repel one another, thus they will be arranged around a central atom as far apart as possible in order to minimize repulsions. This is known as:
Valence Shell Electron Pair Repulsion theory, or VSEPR theory.
The following VSEPR table supplies the names, sketches and descriptions of the most common types of molecular shapes that you will encounter. Note that several other molecular geometries do exist, however, they are beyond the scope of this course.
Electronegativity and Bond Polarity
Some atoms in molecules have the ability to pull shared electrons closer to themselves than other atoms, an ability referred to as electronegativity. Electronegativity (\(\Chi\)) increases going across a period and decreases going down a group.
If two bonded atoms have different electronegativities, then the bond pair electrons will be unequally shared. The atom with the greater electronegativity will pull the bond electrons closer towards itself, causing it to obtain a very slight negative charge (\(\delta ^-\)). The atom with the lower electronegativity will have bond electrons pulled further away from it, causing it to obtain a very slight positive charge (\(\delta ^+\)). The result is a polar covalent bond. However, if two bonded atoms have the same electronegativity, then the bond pair electrons will be equally shared. The result is a non-polar covalent bond.
Example:
The bond electrons are pulled closer to Cl due to its greater electronegativity. Thus, HCl contains a polar covalent bond.
Molecular Polarity
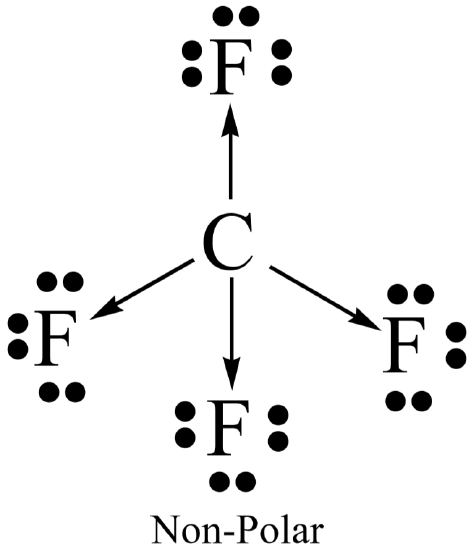
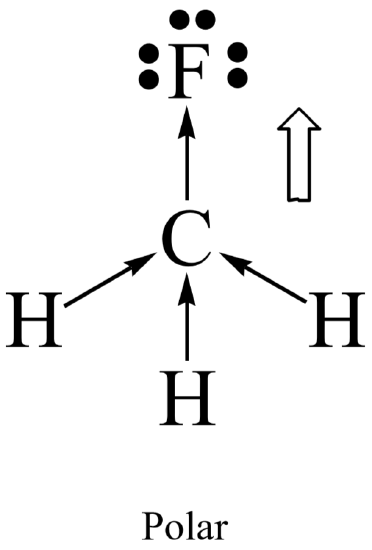
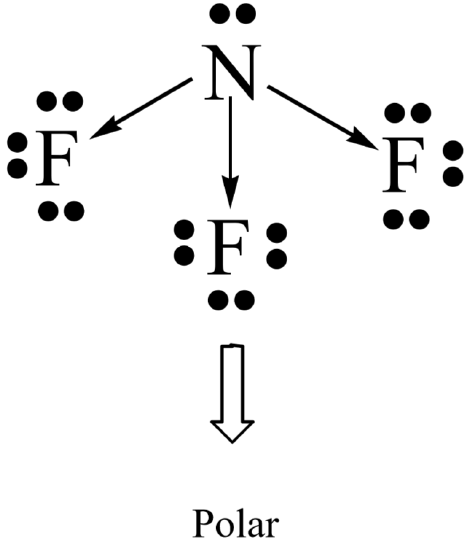
Molecular polarity results when the entire molecule (not just a bond in the molecule) ends up with an unequal distribution of electrons. In general, a molecule will be polar if it contains polar bonds that are distributed in a non-symmetrical arrangement around the central atom. A polar molecule is said to have a net dipole moment. A non-symmetrical arrangement typically results when there are lone pairs on the central atom, or, when different outer atoms surround the central atom.
Not surprisingly, a molecule will be non-polar if it contains all non-polar bonds. It will also be non- polar if it contains polar bonds distributed in a symmetrical arrangement around the central atom. The symmetry causes the individual bond polarities to cancel out, resulting in a net non-polar molecule. A symmetrical arrangement typically results when there are no lone pairs on the central atom, and if all the outer atoms are identical.
Procedure
Drawing Lewis Structures
Draw Lewis structures for each of the molecules and polyatomic ions given on your Report Form. Clearly show all bond pair electrons as lines and lone pair electrons as pairs of dots.
Rules for Drawing Lewis Structures
- Total the number of valence electrons that each atom contributes to the molecule/polyatomic ion.
- For polyatomic anions, you must add electrons (equal to the negative charge) to the total number of valence electrons. For polyatomic cations, you must subtract electrons (equal to the positive charge) from the total number of valence electrons.
- Draw a stick structure for the molecule.
- The least electronegative atom is the central atom. Hydrogen is the only exception to this, as it forms only 1 bond. The central atom will usually need to form multiple bonds.
- The other atoms are arranged around the central atom, and are attached to it via single bonds.
- The octet rule must be obeyed for all elements except hydrogen (follows a “duet” rule).
- Starting with the outside atoms, place the remaining electrons around each atom until it has a total of 8 electrons (except hydrogen – it only requires 2 electrons).
- If there are not enough electrons available to obey the octet rule using single bonds, this indicates that double or triple bonds between two atoms are required in your structure. If short by two electrons, try a double bond, and if short by four electrons, try a triple bond or two double bonds.
Constructing Models, Determining Molecular Shapes and Molecular Polarity
- Use your molecular model kit to construct a three-dimensional model of each of these molecules and polyatomic ions. Sketch a reasonably detailed picture of this model on your Report Form.
Rules for Constructing Molecules with the Model Kit
- Use the short sticks for single bonds.
- Use two long flexible sticks for a double bond and three long flexible sticks for a triple bond.
- Evaluate the polarity of the bonds in each molecule as well as its overall symmetry in order to determine whether it is polar or non-polar.
Lab Report: Lewis Structures and Molecular Shapes
- \(\ce{CH4}\)
|
Total # of Valence Electrons: |
3-D Model Sketch (show dipole arrows) |
|
Lewis Structure
|
|
|
Is there a polar bond in this molecule? |
VSEPR shape name: Molecule Polarity: Polar Non-Polar Resonance: Yes No |
- \(\ce{CO2}\)
|
Total # of Valence Electrons: |
3-D Model Sketch (show dipole arrows) |
|
Lewis Structure
|
|
|
Is there a polar bond in this molecule? |
VSEPR shape name: Molecule Polarity: Polar Non-Polar Resonance: Yes No |
- \(\ce{NH4}\)
|
Total # of Valence Electrons: |
3-D Model Sketch (show dipole arrows) |
|
Lewis Structure
|
|
|
Is there a polar bond in this molecule? |
VSEPR shape name: Molecule Polarity: Polar Non-Polar Resonance: Yes No |
- \(\ce{H2O}\)
|
Total # of Valence Electrons: |
3-D Model Sketch (show dipole arrows) |
|
Lewis Structure
|
|
|
Is there a polar bond in this molecule? |
VSEPR shape name: Molecule Polarity: Polar Non-Polar Resonance: Yes No |
- \(\ce{N2}\)
|
Total # of Valence Electrons: |
3-D Model Sketch (show dipole arrows) |
|
Lewis Structure
|
|
|
Is there a polar bond in this molecule? |
VSEPR shape name: Molecule Polarity: Polar Non-Polar Resonance: Yes No |
- \(\ce{SO2}\)
|
Total # of Valence Electrons: |
3-D Model Sketch (show dipole arrows) |
|
Lewis Structure
|
|
|
Is there a polar bond in this molecule? |
VSEPR shape name: Molecule Polarity: Polar Non-Polar Resonance: Yes No |
- \(\ce{O2}\)
|
Total # of Valence Electrons: |
3-D Model Sketch (show dipole arrows) |
|
Lewis Structure
|
|
|
Is there a polar bond in this molecule? |
VSEPR shape name: Molecule Polarity: Polar Non-Polar Resonance: Yes No |
- \(\ce{O3}\) - use yellow ball for central atom
|
Total # of Valence Electrons: |
3-D Model Sketch (show dipole arrows) |
|
Lewis Structure
|
|
|
Is there a polar bond in this molecule? |
VSEPR shape name: Molecule Polarity: Polar Non-Polar Resonance: Yes No |
- \(\ce{CO}\)
|
Total # of Valence Electrons: |
3-D Model Sketch (show dipole arrows) |
|
Lewis Structure
|
|
|
Is there a polar bond in this molecule? |
VSEPR shape name: Molecule Polarity: Polar Non-Polar Resonance: Yes No |
- \(\ce{CO3^{-2}}\)
|
Total # of Valence Electrons: |
3-D Model Sketch (show dipole arrows) |
|
Lewis Structure
|
|
|
Is there a polar bond in this molecule? |
VSEPR shape name: Molecule Polarity: Polar Non-Polar Resonance: Yes No |
- \(\ce{NO3^-}\) – use a blue ball for phosphorus when constructing this model
|
Total # of Valence Electrons: |
3-D Model Sketch (show dipole arrows) |
|
Lewis Structure
|
|
|
Is there a polar bond in this molecule? |
VSEPR shape name: Molecule Polarity: Polar Non-Polar Resonance: Yes No |
- \(\ce{CF2Cl2}\) (\(\ce{CFC}\) = chlorofuorocarbon)
|
Total # of Valence Electrons: |
3-D Model Sketch (show dipole arrows) |
|
Lewis Structure
|
|
|
Is there a polar bond in this molecule? |
VSEPR shape name: Molecule Polarity: Polar Non-Polar Resonance: Yes No |
Questions
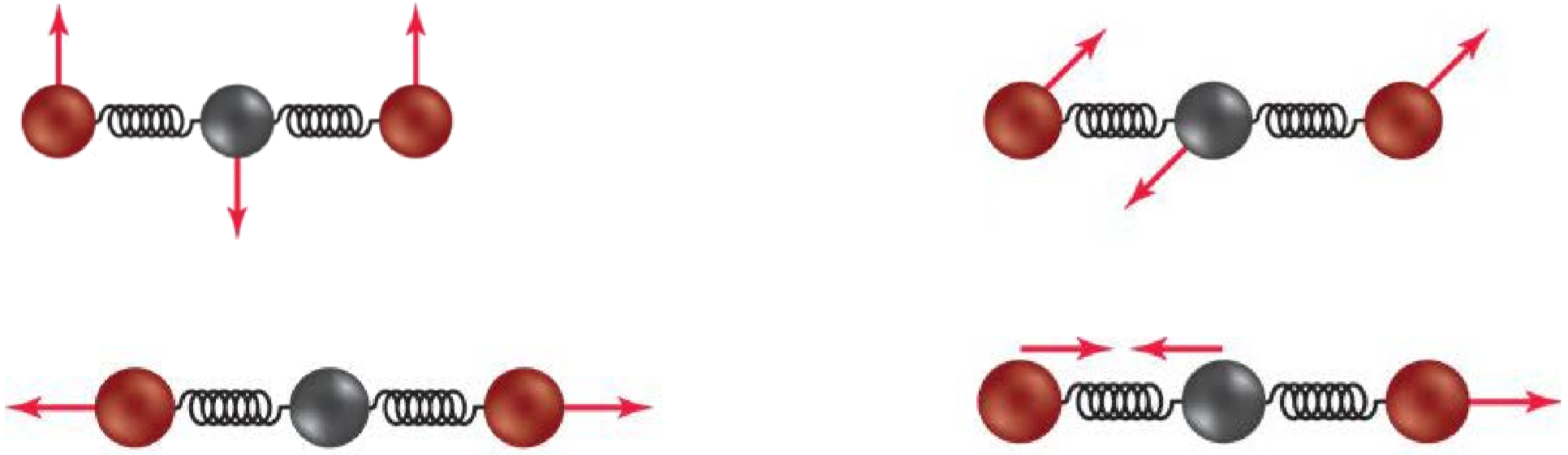
- When molecules and energy interact, there are different results. Atoms may dissociate, a molecule may rotate, or bonds may stretch and bend. Carbon dioxide has four possible vibrations. What is the effect of each vibration on the molecule: stretching, bending, or breaking?
- What is the definition of a greenhouse gas?
- Various atmospheric gases are listed below:
| Chemical Formula | VSEPR Shape (Sketch) | Greenhouse Gas (yes or no) | |
|---|---|---|---|
| carbon dioxide | |||
| methane | |||
| nitrogen | |||
| oxygen | |||
| ozone | |||
| sulfur dioxide | |||
| water |