4: Detection and Absorption of Ultraviolet Light (Experiment)
- Page ID
- 93985
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\(\newcommand{\ket}[1]{\left| #1 \right>} \)
\( \newcommand{\bra}[1]{\left< #1 \right|} \)
\( \newcommand{\braket}[2]{\left< #1 \vphantom{#2} \right| \left. #2 \vphantom{#1} \right>} \)
\( \newcommand{\qmvec}[1]{\mathbf{\vec{#1}}} \)
\( \newcommand{\op}[1]{\hat{\mathbf{#1}}}\)
\( \newcommand{\expect}[1]{\langle #1 \rangle}\)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- To understand what ultraviolet (UV) light is
- To understand the different types of UV light
- To test the abilities of materials to absorb UV light
- To understand relationship between UV light and sun protection factor (SPF)
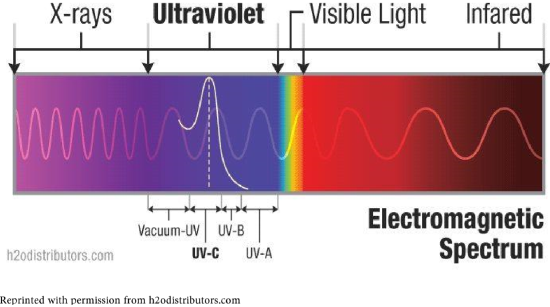
Solar energy (sunlight) contains light we can see, and some we cannot. Visible light has wavelengths of 750 to 400 nm. Ultraviolet (UV) light has shorter wavelengths, cannot be seen, and has higher energy. Infrared (IR) radiation is the major source of heat for Earth. Though UV is a fraction of sunlight, it can be damaging to living organisms. All of these are forms of energy in the electromagnetic spectrum.
Just as visible light components have names (red, orange, yellow, green, blue, indigo, violet), so do the types of UV light: UV-A, UV-B, UV-C and vacuum-UV. UV-A has lowest energy and is least damaging; UV-A is also called “black light.” UV-B and UV-C have higher energies and can cause break bonds of molecules, causing changes in DNA and thus skin cancers.
The majority of UV-B is absorbed by ozone in the stratosphere. Though UV-C is most damaging, it is totally absorbed by oxygen and ozone. In recent years, depletion of the ozone layer has allowed more UV light to reach us, resulting in more cases of skin cancers. Consequently, we have become aware of the need to protect ourselves from UV light.
What protects us from UV light? One strategy would be to avoid exposure to any type of sunlight. Since we cannot avoid sunlight while outdoors, we can physically or chemically block the sun. A wide variety of commercial sunscreens are available with sun protection factors (SPF) ranging from SPF 2 to SPF 100. These lotions contain organic molecules that absorb UV light. Some materials, such as glass and plastic also absorb UV light, while still allowing visible light through.
|
UV light type |
Wavelength |
Relative Energy |
Comments |
|---|---|---|---|
|
UV-A |
320 – 400 nm |
lowest energy |
reaches Earth in greatest amount |
|
UV-B |
280 – 320 nm |
higher energy than UV-A, but less than UV-C |
most is absorbed by ozone |
|
UV-C |
200 – 280 nm |
highest energy |
absorbed by ozone and oxygen |
Procedure
Materials and Equipment
UV-sensitive beads, sunscreen lotions with various SPF ratings, sunblock, sunglasses, clear and opaque plastic, glass plate, foil, cloth, small plastic bags, laboratory UV light, UV intensity meter card
Your eyes should not be directly exposed to the indoor lab UV light source. No materials used in this experiment should be ingested.
Personal protective equipment (PPE) required: lab coat, safety goggles
Part A: Detecting UV light
- Place 3-5 UV-sensitive beads in two small plastic bags. These beads will turn color in the presence of UV light. The higher the intensity of UV light the stronger the color change.
- Make two bags: one labeled “Control” and one labeled “Experiment”. Record the color of the beads when exposed to indoor light (lighting in the lab). The control bag will remain at your bench, and should be used for comparison to color changes in experiment.
- Take the experiment bag outside, in an area of direct sunlight. Record the color of the beads after 10 seconds of exposure to outside sunlight.
- Find a shaded area (e.g. under a tree or in a corridor). Record the color of the beads in shade.
Part B: Absorption of Outdoor UV light by various materials
- Place on a tray: control and experiment bags from Part A and one of each of the materials from the front bench (clear plastic, opaque plastic, sunglasses, glass plate, foil and cloth). Take the tray outside to an area of direct sunlight.
- Use each type of material to block the sunlight by holding directly above the experiment bag. Record the color of the beads when shielded by each sample. Note the colors of both control and experiment beads.
Part C: Absorption of UV Light by lotions
- Place 3-5 UV-sensitive beads into a small plastic bag; make four bags. Label with the SPF numbers, and one sunblock. Note the color of the beads under indoor lighting.
- Add one small drop/dab of each lotion onto each bag. Lightly coat/spread to cover one side of the bag. Allow lotion to dry. Bring the bags to a laboratory UV light setup. Leave bag for 10 seconds. Look into the front of the setup (but not directly at the light) to record the color of the beads under UV light.
- Empty the beads into beaker at front bench. Discard coated plastic bags.
Part D: UV Light at SMC
1. Obtain a UV Intensity Meter card from the front bench. Go outside to the same area of sunlight you had been working in previously. Record the reading on your report sheet.
Lab Report: Detection and Absorption of Ultraviolet Light
Experimental Data and Observations
Part A: Detecting UV Light
|
Condition |
Color of Control Beads |
Color of Experiment Beads |
|---|---|---|
|
indoor light |
||
|
sunlight |
||
|
shade |
Part B: Absorption of UV Light by various materials
|
Condition |
Color of Control Beads |
Color of Experiment Beads |
|---|---|---|
|
translucent (clear) plastic |
||
|
opaque plastic |
||
|
cloth |
||
|
foil |
||
|
glass plate |
||
|
sunglasses |
Part C: Absorption of UV Light by lotions
|
Condition |
Color of Beads Before UV |
Color of Beads Under UV |
|---|---|---|
|
sunblock |
||
|
SPF 50 |
||
|
SPF 15 |
||
|
SPF 5 |
Part D: UV Light at SMC
- Record the intensity warning value, for the UV energy, using the UV Intensity Meter Card:
- Record the warning value and protection advisory (refer to Table 2.5 in Chemistry in Context) that corresponds to the value:
Questions
- What is the difference between UV-A, UV-B, and UV-C light?
- Compare the energies of UV light to IR and visible light. Explain why UV light is potentially more dangerous than IR or visible light.
- Opacity is the opposite of translucent (clear); for a completely opaque substance, you cannot see through it at all. Considering results from the plastics, glass, foil, and cloth pieces, did the opacity of each substance affect ability to block UV light? How might you use this knowledge to protect yourself when outdoors in sunlight?
- What is the purpose of an experimental control? Give one specific example of a control used in this experiment.
- Most sunscreen lotions claim to protect against UV-A and UV-B. Why don’t they mention UV-C light?
- From your data in Part C, do higher SPF ratings provide more protection from UV light?
- If the L.A. Times reports a UV Index of 6.5, and taking into account your skin pigmentation, how might this affect your plans for the day?