Low Solubility Salts in Dairy Products: Calcium Phosphate and Lactate
- Page ID
- 50904
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)


Cow's milk contains about 1 200 mg/L,[1] which makes dairy products a good dietary source of this mineral. In milk, calcium is found as calcium hydrogen phosphate (CaHPO4) associated with casein (the main milk protein) in aggregates called micelles. This colloidal form of calcium hydrogen phosphate is amorphous and varies in composition containing citrate, potassium, sodium, and magnesium. The Ksp of CaHPO4 is 1 x 10–7 which accounts for a solubility of 3.16 x 10–4 mol L–1. However, in spite of its low solubility, CaHPO4 saturates milk due to its association with casein and citrate. [2]

The solubility of calcium salts is highly dependent on pH and changes on this parameter during processing or storage of foods can favor or prevent their precipitation. For example, tricalcium phosphate, presents the following solubility equilibrium
\[\text{Ca}_{3}(\text{PO}_{4})_{2}\text{ }({s})\rightleftharpoons \text{3Ca}^{2+} ({aq}) + \text{2PO}_{4}^{3-} ({aq})\]
The solubility product constant for this equilibrium or Ksp is 2.0 × 10–29 mol5 dm–15, which results in a solubility equal to 7.13 × 10–7 mol dm–3.
If acid is added to this solution, some of the phosphate ions become protonated and transformed into HPO4– ions.
\[\ce{PO4^(3-) + H3O+ <-> HPO4- +H2O } \nonumber\]
As a result, the concentration of the phosphate ion is reduced. According to the Le Chatelier’s principle, the system will respond to this reduction by trying to produce more phosphate ions. Some solid Ca3(PO4)2 will dissolve, and the equilibrium will be shifted to the right. If enough acid is added, the phosphate-ion concentration in the solution can be reduced to make the ion product (Q = cCa2+ × cPO43–) smaller than the solubility product Ksp so that the precipitate dissolves.
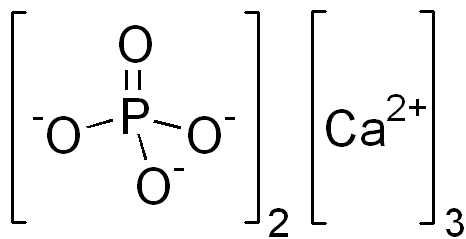
The increased solubility of calcium phosphates at low pH is extremely important in dairy products. The stoichiometry of colloidal calcium hydrogen phosphate (CaHPO4) is more complex than that of tricalcium phosphate and in milk occur not only one main equilibrium, but multiple pseudoequlibria involving calcium phosphates and other components of milk. However, when the pH of milk is lowered, either by direct addition of acid or by fermenting bacteria, calcium hydrogen phosphate dissolves and leaches out from the casein micelle. The loss of calcium weakens the micelle structure and leaves it more sensitive to aggregation. This is in fact one of the first changes occurring in milk during the production of cottage cheese and yogurt.

Formation of calcium lactate crystals is a defect commonly found in cheese. Ingestion of calcium lactate is not harmful to the consumer. However, the crystals appearing as white spots on the surface of the cheese tend to be confused with mold growth and are perceived as a quality defect.
Factors associated with the formation of calcium lactate crystals in Cheddar cheese include high concentration of calcium and lactate in the serum phase of cheese, levels of citrate, and temperatures at different stages of the process. [3] The existence of a single nucleation site,[4] as well as conversion of L(+)-lactate into a racemic mixture of L(+)-lactate and D(-)-lactate by certain strains of lactobacilli and pediococci[5] have also been associated with the crystallization of calcium lactate in cheese. A different study[6] concluded that the starter culture was a major factor determining the development of calcium lactate crystals. Among factors such as pH, lactic acid concentration, calcium (total and soluble), non-protein nitrogen, and salt, a particular profile on lactic acid production and hence lowering of pH was significant in calcium lactate crystal formation. This profile resulted in the least pronounced change in pH.
Another defect tightly related to calcium lactate crystallization is the so called "weeping", which is the expulsion of liquid out from the cheese matrix. This phenomenon is usually observed prior to crystal formation. The protein in cheese has the ability to bind water, this ability is strongly affected by pH and salt.[6] Low pH will on one side favor the leaching of calcium associated with casein into the serum phase as well as modify the charge and conformation of the protein. Both phenomena result in shrinking of protein, loss of water binding capacity, and expulsion of liquid.
The liquid expelled from the cheese matrix is concentrated in both calcium and lactate ions setting the appropriate conditions for crystallization of calcium lactate. Ultimately, the formation of calcium lactate crystals will occur when both calcium and lactate ions are found in concentrations resulting in a Q above the Ksp of calcium lactate and we would expect any condition influencing such concentrations to result in crystallization calcium lactate. In this way we can explain why smoked Cheddar cheese displays calcium lactate crystals so often. Among other changes, during smoking, the surface of the cheese dries creating gradients of moisture and concentrating solutes on the surface of the cheese including calcium and lactate ions. [7]
Besides pH, controlling the salt content in cheese could be a means to prevent both weeping and the formation of calcium lactate crystals. Salt modifies the water binding capacity of protein and can modulate the growth and subsequent production of acid by lactic acid bacteria.[6]
This is an interesting example where the solubility of a calcium compound is increased by a shift of pH to be transformed from a colloidal state as part of an aggregate into a soluble state in the serum fraction of cheese. Then, when it is concentrated enough, the soluble calcium combines with lactate returning to a low solubility state.
A similar effect can be achieved by using a sequestering anion for calcium, such as citrate. Binding of calcium-ions to citrate will decrease the concentration of "free" calcium-ions. The equilibrium will then shift to the left with further dissolution of Ca3(PO4)2. Both pH control and sequestering agents are strategies used to maintain calcium ions in solution in the formulation of foods fortified with calcium.
More Salts of Weak Acids and Exceptions
Other precipitates involving basic anions show similar behavior in acidic pH. These are precipitates in which the anion is basic; i.e., they are the salts of weak acids. Virtually all the carbonates, sulfides, hydroxides, and phosphates which are sparingly soluble in water can be dissolved in acid. Thus, for instance, we can dissolve precipitates like ZnS, Mg(OH)2, and Ca2(PO4)3 because all the following equilibria can be shifted to the right by attacking the basic species S2–, OH–, and CO32– with hydronium ions.
\[\ce{ZnS(s) <-> Zn^(2+)(aq) + S^(2-)(aq)} \nonumber\]
\[\ce{Mg(OH)2 (s) <-> Mg(2+) (aq) + 2OH- (aq)} \nonumber\]
\[\ce{CaCO3 (s) <-> Ca(2+) (aq) + CO3 (2-) (aq)} \nonumber\]
Even though low pH can favor the solubility of salts of weak acids, very occasionally we find an exception to this rule. Mercury(II) sulfide, HgS, is notorious for being insoluble. The solubility product for the equilibrium
\[\ce{HgS (s) <-> Hg(2+) (aq) + S(2-) (aq)} \nonumber\]
is so minute (4 x 10-53) that not even concentrated acid will reduce the sulfide ion sufficiently to make Q smaller than Ksp.
Undesirable effects
There are cases where the shift in a solubility-product equilibrium caused by a decrease in pH may be undesirable. One example of this is the so called acid rainfall, which can occur when oxides of sulfur and other acidic air pollutants are removed from the atmosphere by its own humidity and rain. In some parts of the United States pH values as low as 4.0 have been observed. These acid solutions dissolve marble and limestone (CaCO3) causing considerable property damage. This is especially true in Europe, where some statues and other works of art have been almost completely destroyed over the last half century. This phenomenon is also responsible for the formation of caves, the erosion of coral reefs, and damage of teeth by acids in foods.
From ChemPRIME: 14.12: The Solubilities of Salts of Weak Acids
References
- ↑ Food Chemistry 3rd Ed. 2004 Belitz, et al.
- ↑ Dairy Science and Technology 2nd Ed. 2006 Walstra,et al.
- ↑ Pearce, K. N., Creamer, L. K., and Gilles, J. 1973. Calcium lactate deposits on rindless Cheddar cheese. N.Z. J. Dairy Sci. Technol. 8:3-7.
- ↑ Morris, H. A., Holt, C., Brooker, B. E., Banks, J. M., and Manson, W. 1988. Inorganic constituents of cheese: Analysis of juice from a one-month-old Cheddar cheese and the use of light and electron microscopy to characterize the crystalline phases. J. Dairy Res. 55:255-268.
- ↑ Johnson, M. E., Riesterer, B. A., and Olson, N. F. 1990. Influence of nonstarter bacteria on calcium lactate crystallization on the surface of Cheddar cheese. J. Dairy Sci. 73:1145-1149.
- ↑ Swearingen, P. A., Adams, D. E., and Lensmire, T. L. 2004. Factors affecting calcium lactate and liquid expulsion defects in Cheddar cheese. J. Dairy Sci. 87:574-582.
- ↑ Rajbhandari, P., Patel, J., Valentine, E., and Kindstedt, P. S. 2009. Chemical changes that predispose smoked Cheddar cheese to calcium lactate crystallization. J. Dairy Sci. 92:3616-3622.
Contributors and Attributions
Ed Vitz (Kutztown University), John W. Moore (UW-Madison), Justin Shorb (Hope College), Xavier Prat-Resina (University of Minnesota Rochester), Tim Wendorff, and Adam Hahn.


