Waste Water Treatment
- Page ID
- 50870
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Precipitation Reactions in Wastewater Treatment
It is estimated that the U.S uses about 408 billion gallons of water per day (1). In the process of being used, whether it be for irrigation, drinking, bathing, or industrial applications, it needs to be cleaned before being the wastewater is used again. This process is called wastewater treatment and consists of the following major steps:
- screening
- sedimentation
- precipitation
- filtration
- absorption
- disinfection
Below is a photograph of a wastewater treatment facility

This article focuses on the precipitation portion of the process (for an overview of the entire process please click here or for a video click here). In precipitation reactions, as noted on the main page, substances that are dissolved in water react to form a solid. The main goal of the precipitation portion of the wastewater treatment process is to remove soluble metal ions and phosphates from water. Before delving into the reactions, it is useful to re-examine the solubility rules (from the CoreChem page for precipitation reactions).
| Soluble in Water | Important Exceptions (insoluble) | Sparingly Soluble in Water | Important Exceptions (soluble) |
|---|---|---|---|
| All Na+, K+, and NH4+ salts | All carbonates and phosphates | Group IA and NH4+ salts | |
| All nitrates and perchlorates | All hydroxides | Group IA, Ba2+, Sr2+ | |
| All acetates | Ag+ | All sulfides | Group IA, IIA |
| All sulfates | Ba2+, Sr2+, Pb2+ | ||
| All chlorides, bromides, iodides | Ag+, Hg22+, Pb2+ |
The following electrolytes are of only moderate solubility in water:
CaSO4, Ca(OH)2, Ag2SO4, KClO4
They will precipitate only if rather concentrated solutions are used.
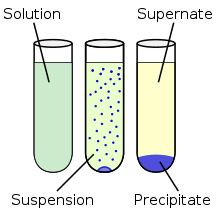
Below is an descriptive image showing a precipitation reaction in a test tube. A solid is formed by the reaction and can then be removed by filtration. This same process holds true in the precipitation reactions in wastewater treatment.
Removal of Ca2+ and Mg2+
The removal of these alkaline earth metals is called water softening. Failure to clean them from the water will not cause health issue, but can lead to chemical deposits throughout the supply chain. In order to remove these ions from the water supply, 2 chemicals are commonly used, lime (Ca(OH)2) and soda ash (Na2CO3). This occurs in the following chemical pathway:
Removal of Mg2+
The soluble lime is now split up in Ca2+ and 2OH- ions and the following chemical reaction occurs:
\[\ce{Mg(2+) (aq) + 2OH- (aq) -> Mg(OH)2 (s)}\]
The calcium ion is a spectator in this case and is not changed.
Removal of Ca2+
There is now the original calcium ions that were present plus the calcium ions added by the introduction of lime. The soda ash is soluble, so it is now comprised of Na+ and CO32- ions. The calcium ions are removed by a precipitation reaction with the carbonate ions as seen below:
\[\ce{Ca(2+) (aq) + CO3(2-) (aq) -> CaCO3 (s)}\]
Removal of Fe2+ and Mn2+
These transition elements are known to cause staining and can promote bacterial growth. The species are oxidized, which causes them to become solid, and are them removed by filtration. Different oxidizing agents (electron acceptors) can be used, the reactions below are specific to potassium permanganate (for iron removal) and chlorine (for manganese removal).
Removal of Fe2+
There are 2 steps in this process. First:
\[\ce{3Fe(2+) (aq) + MnO4- (aq) + 2H2O (l) -> 3Fe(3+) (aq) + MnO2 (s) + 4OH- (aq)}\]
At high pH values, the Fe3+ and OH- then react:
\[\ce{Fe(3+) (aq) + 3OH- (aq) -> Fe(OH)3 (s)}\]
Removal of Mn2+
Like iron removal, there are 2 steps. First:
\[\ce{Mn(2+) (aq) + Cl2 (aq) -> Mn(4+) (aq) + 2Cl- (aq)}\]
(note: this is done at high pH)
The Mn4+ forms insoluble salts with many different anions, but it can be easily removed by the addition of oxygen
\[\ce{Mn(4+) (aq) + O2 (g) -> MnO2 (s) }\]
Removal of Phosphates:
Like in the case of magnesium ion removal, phosphates can be removed from the public water supply with the additon of lime. As lime dissolves it increases the pH due to the addition of hydroxide ions and it produces Ca2+ ions. These calcium ions react at high pH with phosphates by the chemical equation below:
\[\ce{10Ca(2+) (aq) + 6PO4(3-) (aq) + 2OH- (aq) -> Ca10(PO4)6(OH)2 (s)}\]
The resulting calcium/phosphate/hydroxide complex can be easily removed by filtration.
From ChemPRIME: 11.2: Precipitation Reactions
References
1- www.nationalatlas.gov/article..._wateruse.html
2- water.me.vccs.edu/courses/ENV149/methods.htm
3- http://www.lenntech.com/phosphorous-removal.htm
Contributors and Attributions
Ed Vitz (Kutztown University), John W. Moore (UW-Madison), Justin Shorb (Hope College), Xavier Prat-Resina (University of Minnesota Rochester), Tim Wendorff, and Adam Hahn.


