Pyrocarbon and Amazonian Dark Earth
- Page ID
- 50876
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)The soils of the Amazon rainforest are amongst the most fertile in the world. The amazing diversity and density of flora are not only important now as a resource to manage conservatively, but in the past, sustaining the region's indigenous cultures.
Though the fertility of the Amazon rainforest is largely due to its natural climate and river basin, ancient cultures have also played an important role in cultivating the soil to its present state. The dark soil known as terra preto do indio or "Amazonian Dark Earth", has found to be at its richest in certain areas where Mesoamerican cultures were known to live[1]. Emigrants from the southern United States to Brazil during the post-Civil war years obtained information from natives as to where the most fertile areas of land were. The soil upon which their farms were built was noted to contain a large amount of ceramics, and was speculated to have been the foundation for Mesoamerican tribes' major cities. Modern research shows that Amazonian Dark Earth is unusually high in pyrogenic carbon, or charcoal, and that the source of this carbon must be anthropogenic (from humans).

Somewhat puzzlingly, anthropologists have deduced, from current locations of ruins and quality of soil, that ancient Mesoamericans' large agricultural fields were not built on the darkest earth, but rather on lighter, ceramic-sparse soil, known as terra mulata. Conversely, their residential and non-agricultural working areas seem to have been located on concentrated dark soil. This means that the people of the past millennium must have created Amazonian Dark Earth for themselves, consciously or unconsciously.
Theories exist as to whether these tribes planned the fertilization of soil with charcoal or whether they were accidental byproducts of fireplaces and kilns. Regardless, the chemical benefits of charcoal are well known[2]. Its fine-grained texture and many pores retain nutrients and water, and because its origins are plant material, it is non-toxic to plants. Most importantly, it will not decompose into carbon dioxide.
Modern Chemistry of Carbon Compounds
The recognition of Amazonian Dark Earth's high quality has led to the industrial production of pyrogenic carbon for agricultural use in all parts of the world. Charcoal is a special type of carbon allotrope and thus must be prepared under certain conditions.
Pyrogenic carbon is produced by heating organic material in the absence of oxygen under very high temperatures. However, heating in the presence of oxygen will lead to new chemical reactions:
\[\ce{C + 1/2O2 -> CO}\label{1}\]
\[\ce{CO + 1/2O2 -> CO2}\label{2}\]
The triple bond in 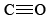 is the strongest chemical bond known, and
is the strongest chemical bond known, and 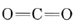 contains two double bonds, and so both molecules are quite stable. Equations \(\ref{1}\) and \(\ref{2}\) occur stepwise when a fuel is burned, and the strong
contains two double bonds, and so both molecules are quite stable. Equations \(\ref{1}\) and \(\ref{2}\) occur stepwise when a fuel is burned, and the strong 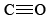 bond makes Eq. \(\ref{2}\) slow unless the temperature is rather high. If there is insufficient O2 or if the products of combustion are cooled rapidly, significant quantities of CO can be produced.
bond makes Eq. \(\ref{2}\) slow unless the temperature is rather high. If there is insufficient O2 or if the products of combustion are cooled rapidly, significant quantities of CO can be produced.
Environmental Impacts of Silicon
Carbon compounds have been shown to have striking diversity in their structures and effects on the environment. As we move down the periodic table, the Group IVA elements become more metallic in nature and thus not as beneficial to the environment as carbon.
Silicon forms polymers similar to that of carbon, though the resulting compounds are much more stiff and obviously inorganic. However, silicon polymers have been recognized since ancient times for their value as ceramics. The Amazonian tribes described in the introduction crafted remarkable pottery from silicon compounds in the earth, using the same kilns that created the carbon-rich Dark Earth. The benefit of these kilns was two-fold, as excavation of ADE soil has uncovered countless buried ceramic chips. These silicon compounds have also been shown to retain a high amount of carbon compounds and water due to their porosity, assisting charcoal in maintaining the richness of the soil.
Ceramics are usually comprised of "silicones". These polymeric substances contain Si—O—Si linkages and may be thought of as derived from silicon dioxide, SiO2. To make silicones, one must first reduce silicon dioxide to silicon. This can be done using carbon as the reducing agent in a high-temperature furnace:
\[\text{SiO}_2 (s) + \text{2C} (s) \xrightarrow []{ \text{3000 deg C}} \text{Si} (l) + \text{2CO} (g)\]
The silicon is then reacted with chloromethane:
\[\text{Si} (s) + \text{2CH}_3\text{Cl} (g) \xrightarrow [\text{Cu catalyst}] {\text{300 deg C}}(\text{CH}_3)_2\text{SiCl}_2 (g)\]
The dichlorodimethylsilane obtained in this reaction polymerizes when treated with water:
n(CH3)2SiCl2 + nH2O → +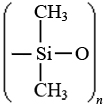 2nHCl
2nHCl
The silicone polymer consists of a strongly bonded —Si—O—Si—O—Si—O chain, called a siloxane chain, with two methyl groups (or other organic groups) on each silicon atom. The strong backbone of a silicone polymer makes it stable to heat and difficult to decompose, traits desirable in pottery. Contemporary museums have utilized radiographs to analyze the chemical composition of ancient pottery and found hard-to-distinguish quartz (a mineral comprised mainly of SiO2) embellishments upon the main silicone frame[3].
From ChemPRIME: 12.4: Group IVA
References
- ↑ s08.cgpublisher.com/proposals/58/index_html
- ↑ http://www.npr.org/templates/story/s...oryId=89562594
- ↑ http://www.penn.museum/sites/biomoleculararchaeology/?page_id=263
Contributors and Attributions
Ed Vitz (Kutztown University), John W. Moore (UW-Madison), Justin Shorb (Hope College), Xavier Prat-Resina (University of Minnesota Rochester), Tim Wendorff, and Adam Hahn.


