Methane and Global Warming
- Page ID
- 50822
Alkanes – Methane’s Impact on Global Warming
Alkanes are compounds where all carbon atoms are linked by single bonds, such as methane (CH4), ethane (C2H6), and propane (C3H8). The alkanes are rather unreactive and do not combine readily with other substances. When heated sufficiently, however, they burn in air, a process known as combustion:
\[\ce{CH4 (g) + 2O2 (g) -> CO2 (g) + 2H2O (g)}\nonumber\]
ΔHm = –890.4 kJ mol-1
Combustion releases large quantities of heat; not surprisingly, the most important use of alkanes is as fuels. Natural gas is mainly (approximately 85 percent) methane.
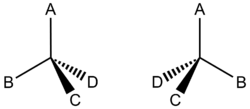
Propane or liquefied petroleum gas sold in tanks for portable use is usually a mixture of both propane and butane. Though both are gases at ordinary temperature and pressure, they liquefy under pressure in the tank. Gasoline is a more complex mixture of alkanes having 5 to 12 carbon atoms. Better-quality gasoline usually contains a higher percentage of branch-chain alkanes than the regular grade. Kerosene contains C10 to C16 alkanes, while heating oil usually involves the C12 to C18 range. Even longer-chain alkanes are found in lubricating oil, C15 to C25 and paraffin wax (used for candles and waxed paper), C23 to C29. The variation of boiling point with chain length in the alkanes provides a simple method for partially separating them from each other in petroleum. When petroleum is heated, the shorter-chain compounds begin to boil off initially. These can be collected by cooling the vapor until it recondenses to a liquid. As boiling proceeds, the temperature rises and longer and longer-chain compounds boil off. Finally only the very long chain compounds are left. Such a process is called fractional distillation and is discussed more in depth in the section on distillation. Fractional distillation plays an important role in petroleum refining, which is a series of physical and chemical processes by which the most valuable and useful components are obtained from natural crude oil. The alkanes are typical examples of nonpolar compounds. The electronegativities of carbon and hydrogen are 2.5 and 2.1, respectively, and so the C—H bond has almost no polarity. Consequently, even the most unsymmetrical hydrocarbon molecules have very small dipole moments. We can therefore take the boiling-point behavior of the alkanes to be representative of other nonpolar substances. Simple molecules are gases, while medium-sized molecules are liquids, and only quite large molecules are solids. Alkanes have carbons with four single bonds. In most examples, these are all to either hydrogen or to other carbon atoms. However, even when carbon only bonds to hydrogen and carbon, we still see a type of stereoisomerism that is different from cis/trans isomerism. Take a look at Figure 1. This molecule demonstrates that if carbon is bonded to four different species, then it is has optical isomers. Optical isomers are two compounds which have the same atoms in the same bonding structure and even can be represented by the same line structure - but have different three-dimensional bonding arrangements in space. A carbon which has four different species attached to it is considered a chiral carbon.
Figure \(\PageIndex{1}\) Optical Isomers
Of the alkanes, it is methane that plays one of the largest roles as a heat trapping molecule when it is released into the atmosphere through both natural and anthropogenic sources.
Natural Sources of Methane: Natural methane emissions come primarily from anaerobic environments such as wetlands, geologic processes, and as a major component of natural gas. Wetlands such as marshes, swamps, and bogs are responsible for the majority of global methane emissions from natural sources. Wetlands provide a habitat conducive to methane-producing bacteria that create methane during the decomposition of organic material. One of the dominant sources of geologic methane is mud volcanoes. These structures can be up to 10 kilometers in diameter, though most are much smaller, and often form on tectonic plate boundaries or near fossil fuel deposits. These natural sources of methane are removed primarily through oxidation by hydroxyl radicals (OH) in the troposphere through a reaction that creates CH3 and water, which leaves anthropogenic sources as the area of the biggest concern. (2)

Anthropogenic Sources of Methane: Increasing levels of atmospheric methane are due mainly to burial of waste in landfills, enteric fermentation in agricultural livestock, rice farming, and the burning of biomass. Methane is generated in landfills and open dumps as waste decomposes under anaerobic conditions. Among domesticated livestock, animals such as cows and sheep produce significant amounts of methane as part of their normal digestive processes. This microbial fermentation process, referred to as enteric fermentation, produces methane as a by-product, which can be exhaled by the animal. Methane is produced during flooded rice cultivation by the anaerobic decomposition of organic matter in the soil. The result of these human activities is anthropogenic sources of methane that are twice as high as natural sources. (1)
For a detailed look at sources of methane on Earth, watch the following video: [1] (5)
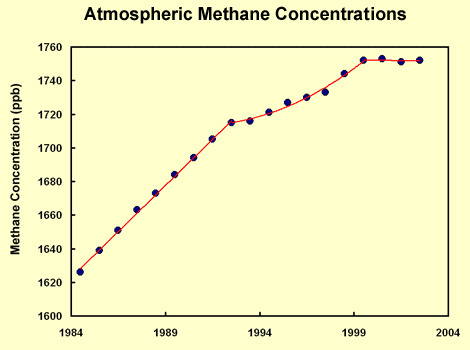
Effects of Methane in the Atmosphere: The atmospheric concentration of methane has increased dramatically in the past one hundred years, and continues to grow. (4) Once in the atmosphere, methane is able to absorb infrared radiation at a rate 21 times greater than the most prolific greenhouse gas carbon dioxide (CO2). (2) Still, because methane persists for only 12 years it is expected to account for only 18% of the total expected global warming compared to the 50% that CO2 is responsible for. (1) But, if you take into consideration the ability of methane to create tropospheric ozone through chemical reactions, it could account for up to a third of recent climate warming. (3) Methane reacting with nitrogen oxides present in the troposphere is capable of creating ozone, which is a beneficial compound in the stratosphere, but a major pollutant in the lowest layer of our atmosphere. In fact, the complete oxidation of a single mole of methane can produce 3 to 4 moles of ozone. (4)Overall, the rate of methane oxidation is very slow, between 100 and 1000 times slower than other organic compounds. This is why methane concentrations in the atmosphere can reach around 1.77 ppm (1.77 µmol mol-1), a value significantly higher than the concentrations of other organic trace gas concentrations present which are generally below 1 ppb (1 nmol mol-1). (6) The combination of concentration, heat trapping ability, and persistence of methane in our atmosphere make it a highly influential greenhouse gas that, if controlled, could result in significant short-term gains in limiting global warming.
From ChemPRIME: 8.7: Properties of Alkanes
References:
1. Milich, Lenard. The Role of Methane in Global Warming: Where might mitigation strategies be focused? Global Environmental Change. Issue 9, Pages 179-201. 1999.
2. U.S. Environmental Protection Agency. epa.gov/methane/scientific.html
3. NASA. www.nasa.gov/vision/earth/lookingatearth/methane.html
4. Wuebbles, Donald J. and Hayhoe, Katharyn. Atmospheric Methane: Trends and Impacts. Accessed 6/12/12 at: http://www.atmosresearch.com/NCGG2a%202002.pdf
5. Methane: The Forgotten Gas. Accessed 7/24/12 at http://www.abc.net.au/catalyst/stories/2373958.htm
6. www.atmosphere.mpg.de/enid/24y.html
• Background information on alkanes obtained from ChorChem information on the ChemPrime website.
Contributors and Attributions
Ed Vitz (Kutztown University), John W. Moore (UW-Madison), Justin Shorb (Hope College), Xavier Prat-Resina (University of Minnesota Rochester), Tim Wendorff, and Adam Hahn.