Environment: Waste Water Treatment
- Page ID
- 50866
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Waste Water Treatment and Separations of Mixtures
Human sewage is a waste product that is unavoidable, but it can be treated to minimize environmental impacts. Sewage treatment is the process of removing impurities mixed in water so that the remaining waste water can be safely returned to the surface waters (river, bay, and ocean) and become part of the natural water cycle again.
Wastewater is a mixture of solids, liquids, and sometimes gases. The wastewater goes through three treatment processes in order to separate substances out of the mixture in order obtain water in the purest form possible. This way it can be returned back to the watershed or even used as drinking water. Since some substances are soluble and others are insoluble different procedures are performed to remove each substance.
According to the ChemCore information on Separations of Mixtures, when a pure substance is mixed with another pure substance in which it is soluble, the substances become completely interspersed at the molecular level. In thinking about making solutions at the molecular level, an analogy to a can of marbles may be useful. In the analogy, a layer of red marbles is placed in the bottom of a can and covered with a second layer of white marbles. After shaking the can for a short time, the marbles are mixed randomly.
Now let us imagine that you want to collect all the red marbles again. If you simply shake the can, it is unlikely that you will ever divide the marbles into two layers, each with only one kind of marble. Similarly, if two miscible liquids are combined, a chemist cannot simply unmix the liquids into pure components.
Continuing the analogy, what if a few green marbles and blue marbles are placed into the can? Given enough red and white marbles, it may be difficult to determine that the green marbles and blue marbles are actually there. Similarly, when chemists have a multi-component solution which may contain traces of important chemical species, they are faced with the challenge of detecting whether these chemicals are present in solution.
To deal with these difficulties, chemists employ different methods to separate solutions into their components. Waste water is treated in three different stages of treatment: primary, secondary, and tertiary treatments. Based on the US Environmental Protection Agency’s Clean Water Needs survey results, in 2000, there were 16, 255 publicly owned wastewater treatment facilities in operation within the US. These facilities served about 75% of the US population, about 207 million people, and treated about 45,058 million gallons of water per day. [1]
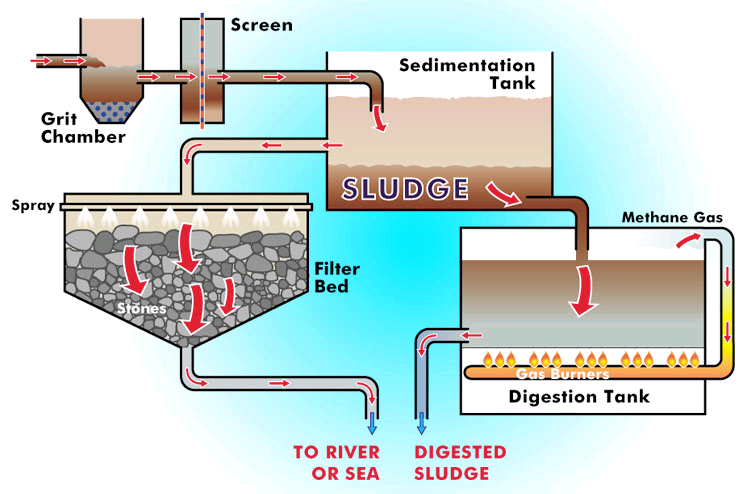
Primary Treatment
The first stage of waste water treatment is known as the preliminary screening. The screening process removes insoluble particles. The smaller particulates and liquid pollutants move on to the primary sedimentation tank. Smaller particulates such as sand and suspended insoluble solids settle out due to gravity. Immiscible liquids in water like grease and oils float to the top and are also removed. The treated waste water is drained off, leaving behind the settled material now called preliminary sludge.
Preliminary sludge can be incinerated or pumped into a digester for decomposition by anaerobic bacteria.
Secondary Treatment
Secondary treatment starts in an aeration tank. Organic materials are broken down by microorganisms into carbon dioxide and water. Air is pumped into the tank, to provide oxygen-rich environment in order to enhance the aerobic decomposition.
Tertiary Treatment
Although most of the organic matter is broken down by the microorganisms in the secondary treatment, substances such as harmful viruses, heavy metals, phosphorous and nitrogen compounds can still present in colloidal solution.
Flocculation
To remove colloidal solids iron (III) sulfate (Fe2 (SO4 )3 ) or aluminum sulfate (Al2 (SO4 )3 ) salts can be added to the solution. These salts are soluable and dissolve and then the cations react to form hydroxide salts. These hydroxides are insoluable so they form a precipitate, or floc, that coagulates with the colloidal solids and drags them to the bottom of the tank. This sedimentation forms a heavy secondary sludge that settles out over time due to gravity. [2]
Disinfection
Remaining bacteria and viruses can be removed through the processes of ozonation, chlorination, or UV light exposure. All three treatments have risks. Ozonation and chlorination can create dangerous by products like formaldehyde, trihalomethanes (THM), and chloroform. UV light exposure can be blocked by particulate matter. Therefore, not all microbes may be exposed to the light and may remain in the water.
The simplest, less toxic form of treating water for microbes is to run the treated waste water through sand.
Aeration
Additional aeration allows for the removal of dissolved gases, such as volatile organic compounds (VOCs) and sulfides. This also removes the rotten egg smell that is commonly affiliated with the sulfides.
According to the United States Department of Agriculture Forest Service Technology and Development Program iron (Fe) is commonly found in water supplies. High levels of iron can stain plumbing fixtures, and create an unpleasant metallic taste and discolor the water. To remove it, in the presence of oxygen, soluble ferrous iron (Fe2+) is oxidized to a ferric iron (Fe3+). The ferric iron readily forms insoluble iron hydroxide Fe(OH)3. The insoluble metals precipitate out of solution and settle at the bottom of the tank due to gravity. [3]
Example \(\PageIndex{1}\): Reaction Products
In an environmental science classroom, a teacher wanted to provide her students with a demo of the process of flocculation. She chose to use sodium hydroxide (NaOH) and magnesium chloride (MgCl). State whether or not a precipitate will form and if so complete the net ionic equation. NaOH (aq) + MgCl2 (aq) -----> ??
Answer
Separate the reactants into their ionic form, as they would exist in an aqueous solution:
\[\ce{Na+ (aq) + OH- (aq) + Mg(2+) (aq) + 2Cl- (aq) -> ??? }\nonumber\]
Based on how a double replacement reaction occurs, one can conclude that the Na and Cl ions would combine while the OH and Mg ions combine. To ensure that this is correct check the solubility of each molecule. The NaCl molecule contains sodium. Sodium is a group 1 cation, it is soluble and therefore will not precipitate based on the first rule of solubility. Mg(OH)2 molecule is a hydroxide; hydroxides are insoluble and will precipitate.
\[\ce{Na+ (aq) + OH- (aq) + Mg(2+) (aq) + 2Cl- (aq) -> Mg(OH)2 (s) + Na+ (aq) + 2Cl- (aq)}\nonumber\]
Be sure balance both the electrical charge and the number of atoms
\[\ce{2Na+ (aq) + 2OH- (aq) + Mg(2+) (aq) + 2Cl- (aq) -> Mg(OH)2 (s) + 2Na+ (aq) + 2Cl- (aq)}\nonumber\]
Example \(\PageIndex{2}\): Solubilty of Hydrocarbons
Based on your knowledge of solubility which hydrocarbon would be the most difficult to remove from waste water. Hint: Predict which of the following compounds will be most soluble in water:
a)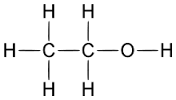 (ethanol)
(ethanol)
b) 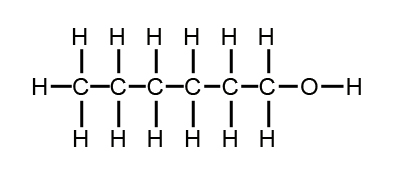 (hexanol)
(hexanol)
Answer
Since ethanol contains an OH group, it can hydrogen bond to water. Although the same is true of hexanol, the OH group is found only at one end of a fairly large molecule. The rest of the molecule can be expected to behave much as though it were a nonpolar alkane. This substance should thus be much less soluble than the first. Experimentally, we find that ethanol is completely miscible with water, while only 0.6 g hexanol dissolves in 100 g water.
From ChemPRIME: 10.20: The Separation of Mixtures
References:
1. "Drinking Water Contaminants." Home. Environmental Protection Agency, 5 June 2012. Web. 12 June 2012.http://water.epa.gov/drink/contaminants/index.cfm
2. "Iron and Manganese In Drinking Water." Iron and Manganese In Drinking Water. United States Department of Agriculture Forest Service,Sept. 1999. Web. 12 June 2012. http://www.fs.fed.us/eng/pubs/html/99711308/99711308.html
3. "Precipitation Reaction." Precipitation Reactions II. UC Davis Chem Wiki, n.d. Web. 12 June 2012.chemwiki.ucdavis.edu/Inorgani...ctice_Problems
Contributors and Attributions
Ed Vitz (Kutztown University), John W. Moore (UW-Madison), Justin Shorb (Hope College), Xavier Prat-Resina (University of Minnesota Rochester), Tim Wendorff, and Adam Hahn.


