Secondary Protein Structure in Silk
- Page ID
- 50932
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
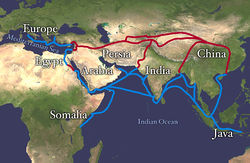
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)There were many trade routes throughout the ancient world. The most highly traveled and culturally significant of these was called the Silk Road. The Silk Road ran from the Chinese city of Chang'an all the way through India and into the Mediterranean and Egypt. The reason that the Silk road was so culturally significant was because of the great distance that it covered. Essentially the entire ancient world was connected by one trade route.

On the route many things were traded, including silk, spices, slaves, ideas, and gun powder. The silk road had an astounding effect on the creation of many societies. It was able to bring economic wealth into areas along the route, and new ideas traveled the distance and influence many things including art. An example of this is Buddhist art that was found in India. The painting has many western influences that can be identified in it, such as realistic musculature of the people being painted. Also, the trade of gun powder to the West helped influence warfare, and in turn shaped the modern world. The real reason the Silk Road was started though was for the product that it takes its name from: Silk.
Silk was prized by the Kings and Queens of both European and Middle Eastern Society. The Silk showed that the rulers had power and wealth because the silk was not easy to come by, and therefore was definitely not cheap. Silk was first developed in China, and is made by harvesting the silk from the cocoons of the mulberry silkworm. The silk itself is called a natural protein fiber because it is composed of a pattern of amino acids in a secondary protein structure. The secondary structure of silk is the beta pleated sheet. The primary structure of silk contains the amino acids of glycine, alanine, serine, in specific repeating pattern. These three amino acids make up 90% of the protein in silk. The last 10% is comprised of the amino acids glutamic acid, valine, and aspartic acid. These amino acids are used as side chains and affect things such as elasticity and strength. they also vary between various species. The beta pleated sheet of silk is connected by hydrogen bonds. The hydrogen bonds in the silk form beta pleated sheets rather than alpha helixes because of where the bonds occur. The hydrogen bonds go from the amide hydrogens on one protein chain to the corresponding carbonyl oxygen across the way on the other protein chain. This is in contrast to the alpha helix because in that structure the bonds go from the amide to the carbonyl oxygen, but they are not adjacent. The carbonyl oxygen is on the amino acid that is four residues before.
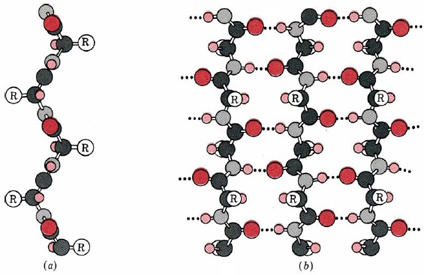
Silk is a great example of a beta pleated sheet. The formation of the secondary protein structure in silk allows it to have very strong tensile strength. Silk also helped to form one of the greatest trading routes in history, allowing for the exchange of ideas, products and cultures while advancing the societies that were involved.
More on Alpha Helices and Beta Sheets
A particularly important conformation of the polypeptide chain is the spiral structure shown in Figure 1. This is called an α helix. Many fibrous proteins like hair, skin, and nails consist almost entirely of α helices. In globular proteins too, although the overall structure is more complex, short lengths of the chain often have this configuration. In an a helix the polypeptide chain is twisted into a right-hand spiral—the chain turns around clockwise as one moves along it. The spiral is held together by hydrogen bonds from the amido (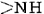 ) group of one peptide bond to the carbonyl group
) group of one peptide bond to the carbonyl group 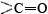 of a peptide bond three residues farther along the chain. Two factors contribute toward making this a particularly stable structure. One is the involvement of all the
of a peptide bond three residues farther along the chain. Two factors contribute toward making this a particularly stable structure. One is the involvement of all the 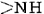 and
and 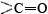 groups in the chain in the hydrogen bonding. Spirals with slightly more or slightly less twist do not permit this. The second factor is the way in which the side chains project outward from an α helix. Bulky side chains therefore do not interfere with the hydrogen bonding, enabling a fairly rigid cylinder to be formed.
groups in the chain in the hydrogen bonding. Spirals with slightly more or slightly less twist do not permit this. The second factor is the way in which the side chains project outward from an α helix. Bulky side chains therefore do not interfere with the hydrogen bonding, enabling a fairly rigid cylinder to be formed.
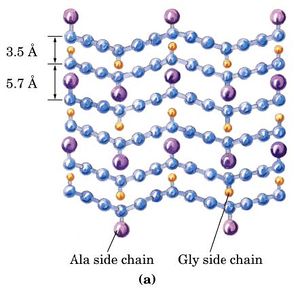
A second regular arrangement of the polypeptide chain is the β sheet, the β-keratin structure found in silk and shown in Figure \(\PageIndex{3}\). As in the α helix, this structure allows all the amido and carbonyl groups to participate in hydrogen bonds. This hydrogen bonding structure can be accomplished in two manners, either a parallel or antiparallel β sheet, which are compared in Figure \(\PageIndex{5}\). Silk contains both anti-parallel and parallel arrangements of beta sheets.
Unlike the α helix, though, the side chains are squeezed rather close together in a pleated-sheet arrangement. In consequence very bulky side chains make the structure unstable. This explains why silk is composed almost entirely of glycine, alanine, and serine, the three amino acids with the smallest side chains. Some species of silk worm produce varying amounts of bulky side chains, but these silks are not as prized as the mulberry silkworm (which has no bulky proteins) because the silk is weaker and doesn't have as much tensile strength. Most other proteins formed contain a much more haphazard collection of amino acid residues.
From ChemPRIME: 20.14: Secondary Protein Structure
References
Contributors and Attributions
Ed Vitz (Kutztown University), John W. Moore (UW-Madison), Justin Shorb (Hope College), Xavier Prat-Resina (University of Minnesota Rochester), Tim Wendorff, and Adam Hahn.


