3.1.1.1: SN2 reactions
- Page ID
- 1401
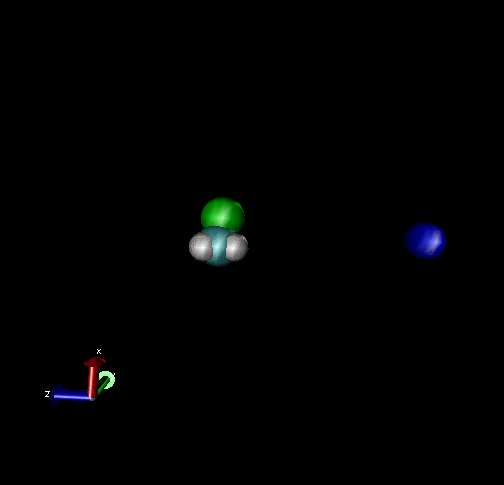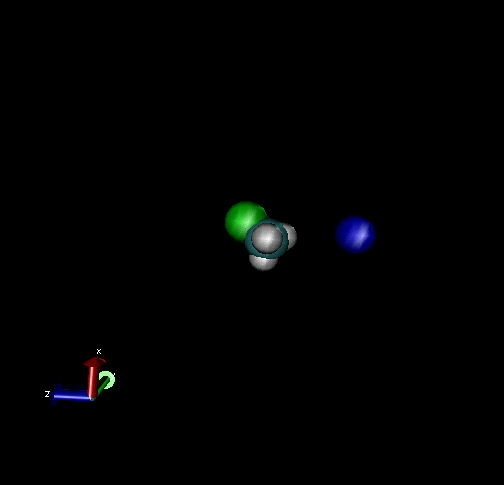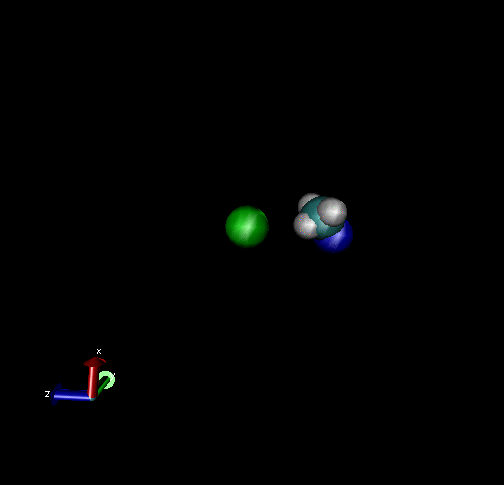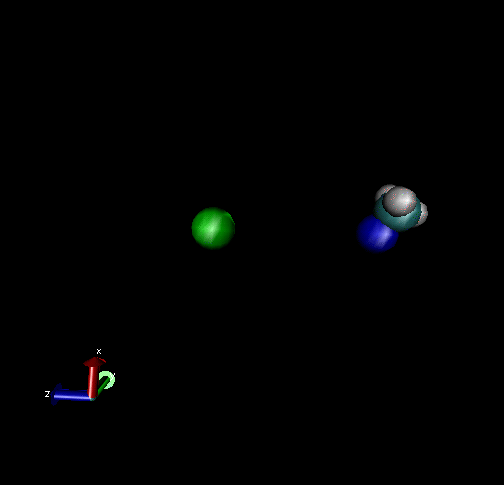
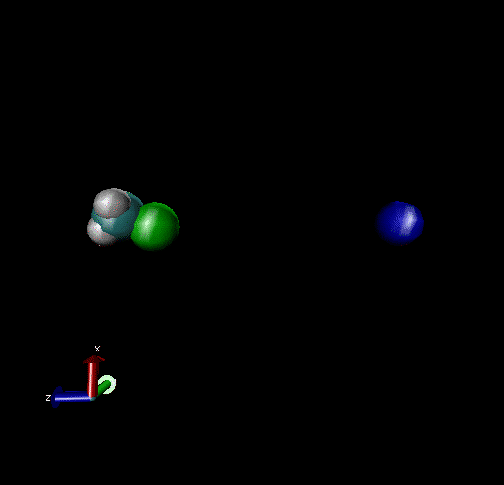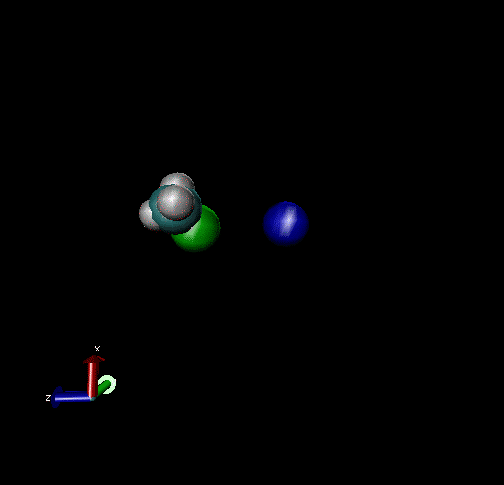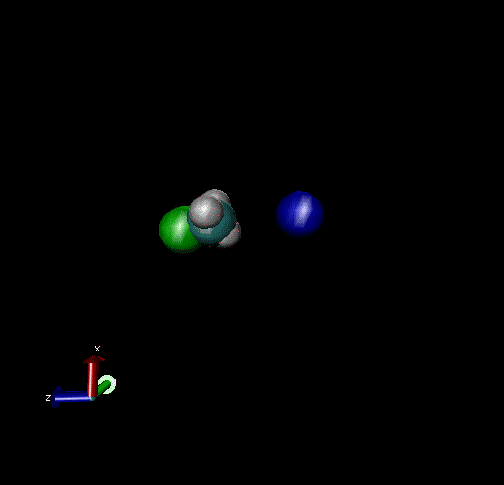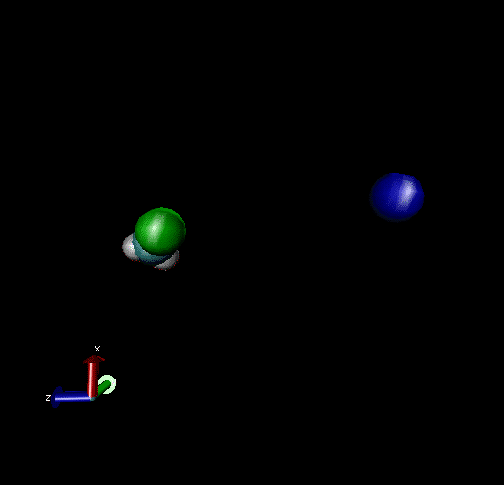
The following are animations of gas-phase trajectories for reactants with different amounts and types of energies. The reactant energy includes a relative translational energy, Erel, between Cl- + CH3Br, and a temperature, Tvr, for the CH3Br vibrational and rotational energies.
Select Trajectories of the Cl- + CH3Br → ClCH3 + Br- SN2 Reaction
|
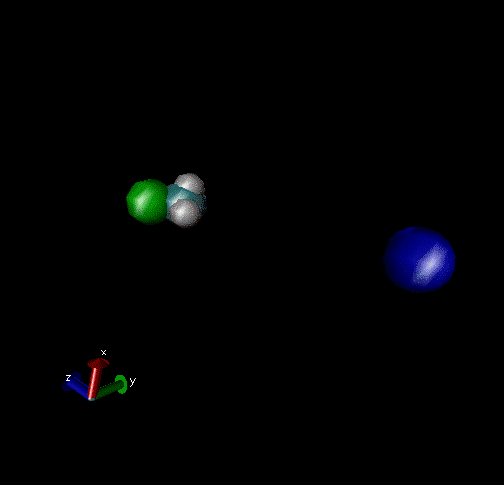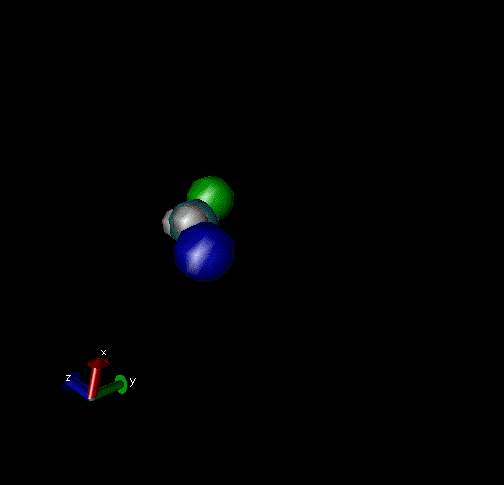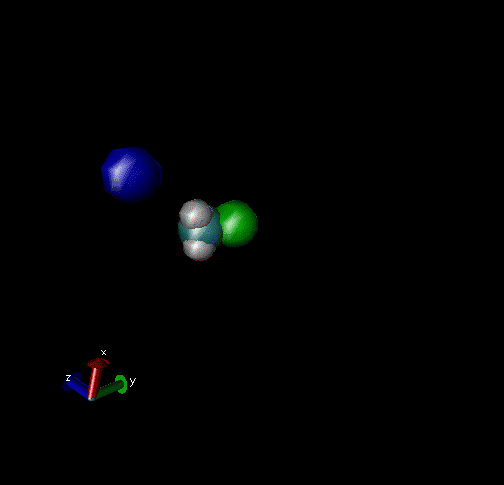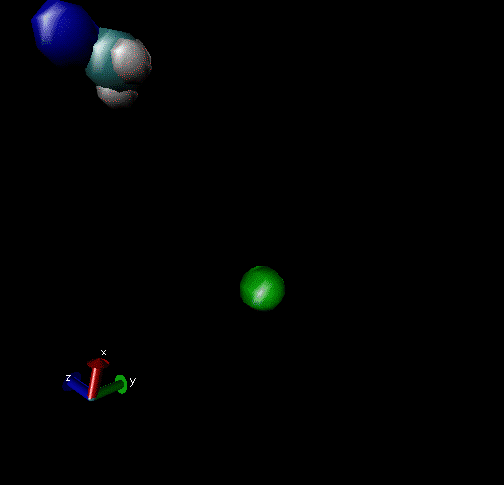
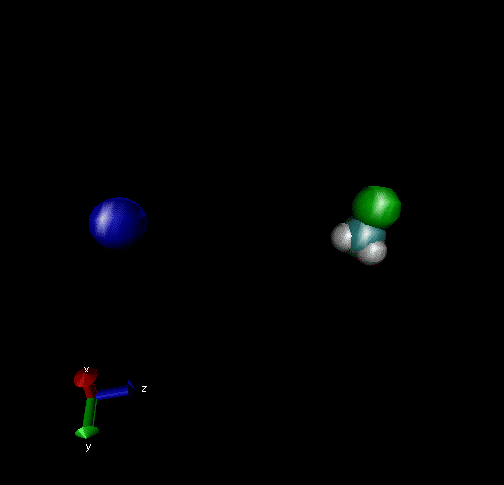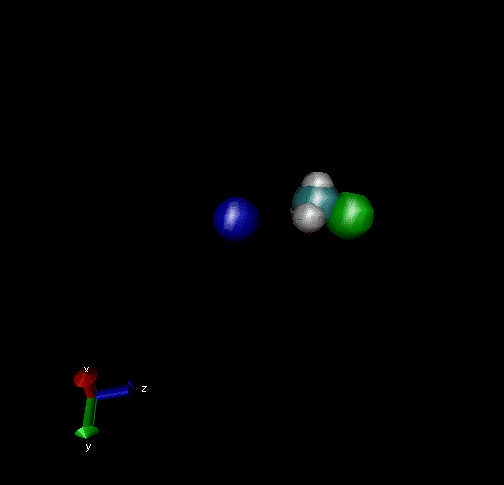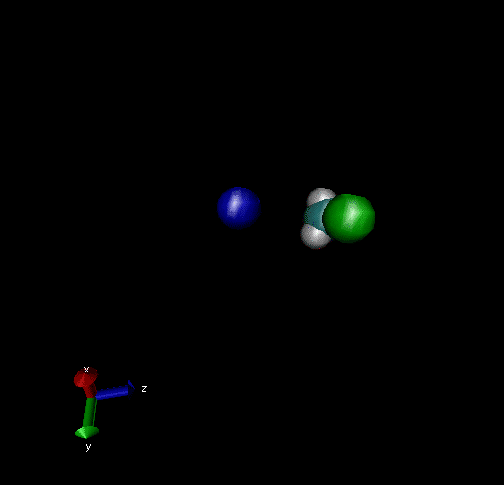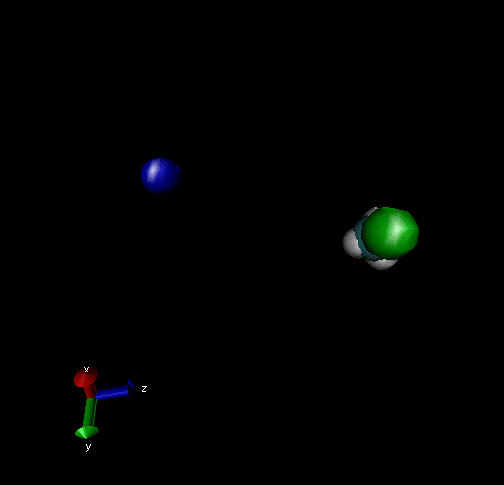
Cl-+ CH3Br → ClCH3 + Br - Nucleophilic substitution by a direct mechanism; Erel = 50 kcal/mol and Tvr = 300K
|
Cl- + CH3Br → Cl----CH3Br → ClCH3Br + Br- Nucleophilic substitution by an indirect mechanism, involving the Cl----CH3 complex; Erel = 1.0 kcal/mol and Tvr = 300K
|
|
Cl- + CH3Br → Cl----CH3Br → ClCH3---Br-→ ClCH3+Br- Nucleophilic substitution by an indirect Reaction mechanism, involving both Cl----CH3Br and ClCH3---Br- complex; Erel = 1.0 kcal/mol and Tvr = 300K
|
Cl- + CH3Br → Cl- + CH3Br A non-reactive collision; Erel = 1.0 kcal/mol and Tvr = 300K
|
|
Cl-+CH3Br → Cl----CH3Br → Cl-+CH3Br A non-reactive collision forming the Cl----CH3Br complex, which dissociates back to reactants; Erel = 1.0 kcal/mol and Tvr = 300K.
|
Cl-+CH3Br → Cl----CH3Br → ClCH3---Br-→ Cl----CH3Br → Cl-+CH3Br Nucleophilic substitution by an indirect mechanism involving both the Cl----CH3Br and ClCH3---Br - complex and recrossing the transition state separating these complexes; Erel = 1.0 kcal/mol and Tvr = 300K.
|
References
Contributors
- Prof. William L. Hase (Department of Chemistry and Biochemistry, Texas Tech University, Lubbock, Texas)