Physical Properties of Alkenes
- Page ID
- 874
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Alkenes contains a carbon-carbon double bond. This carbon-carbon double bond changes the physicals properties of alkenes. At room temperatue, alkenes exist in all three phases, solid, liquids, and gases. Melting and boiling points of alkenes are similar to that of alkanes, however, isomers of cis alkenes have lower melting points than that of trans isomers. Alkenes display a weak dipole-dipole interactions due to the electron-attracting sp2carbon.
Alkenes are a family of hydrocarbons (compounds containing carbon and hydrogen only) containing a carbon-carbon double bond. The first two are:
- ethene ( \(C_2H_4\) )
- propene (\(C_3H_6 \))
You can work out the formula of any of them using: \(C_nH_{2n}\). The list is limited to the first two, because after that there are isomers which affect the names.
Structural Isomerism
All the alkenes with 4 or more carbon atoms in them show structural isomerism. This means that there are two or more different structural formulae that you can draw for each molecular formula. For example, with C4H8, it isn't too difficult to come up with these three structural isomers:
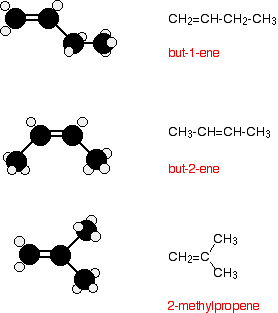
There is, however, another isomer. But-2-ene also exhibits geometric isomerism.
Geometric (cis-trans) Isomerism
The carbon-carbon double bond doesn't allow any rotation about it. That means that it is possible to have the CH3 groups on either end of the molecule locked either on one side of the molecule or opposite each other. These are called cis-but-2-ene (where the groups are on the same side) or trans-but-2-ene (where they are on opposite sides).
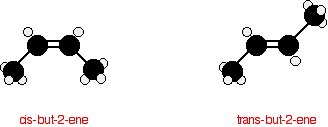
Cis-but-2-ene is also known as (Z)-but-2-ene; trans-but-2-ene is also known as (E)-but-2-ene. For an explanation of the two ways of naming these two compounds.
Physical state
Ethene, Propene, and Butene exists as colorless gases. Members of the 5 or more carbons such as Pentene, Hexene, and Heptene are liquid, and members of the 15 carbons or more are solids.
Density
Alkenes are lighter than water and are insoluble in water due to their non-polar characteristics. Alkenes are only soluble in nonpolar solvents.
Solubility
Alkenes are virtually insoluble in water, but dissolve in organic solvents. The reasons for this are exactly the same as for the alkanes.
Boiling Points
The boiling point of each alkene is very similar to that of the alkane with the same number of carbon atoms. Ethene, propene and the various butenes are gases at room temperature. All the rest that you are likely to come across are liquids. Boiling points of alkenes depends on more molecular mass (chain length). The more intermolecular mass is added, the higher the boiling point. Intermolecular forces of alkenes gets stronger with increase in the size of the molecules.
| Compound | Melting Points (°C) | Boiling points (°C) |
|---|---|---|
| Ethene | -169 | -104 |
| Propene | -185 | -47 |
| Trans-2-Butene | 0.9 | |
| Cis-2-butene | 3.7 | |
| Trans 1,2-dichlorobutene | 155 | |
| Cis 1,2-dichlorobutene | 152 | |
| 1-Pentene | -165 | 30 |
| Trans-2-Pentene | -135 | 36 |
| Cis-2-Pentene | -180 | 37 |
| 1-Heptene | -119 | 115 |
| 3-Octene | -101.9 | 122 |
| 3-Nonene | -81.4 | 147 |
| 5-Decene | -66.3 | 170 |
In each case, the alkene has a boiling point which is a small number of degrees lower than the corresponding alkane. The only attractions involved are Van der Waals dispersion forces, and these depend on the shape of the molecule and the number of electrons it contains. Each alkene has 2 fewer electrons than the alkane with the same number of carbons.
Melting Points
Melting points of alkenes depends on the packaging of the molecules. Alkenes have similar melting points to that of alkanes, however, in cis isomers molecules are package in a U-bending shape, therefore, will display a lower melting points to that of the trans isomers.
Polarity
Chemical structure and fuctional groups can affect the polarity of alkenes compounds. The sp2 carbon is much more electron-withdrawing than the sp3 hybridize orbitals, therefore, creates a weak dipole along the substituent weak alkenly carbon bond. The two individual dipoles together form a net molecular dipole. In trans-subsituted alkenes, the dipole cancel each other out. In cis-subsituted alkenes there is a net dipole, therefore contributing to higher boiling in cis-isomers than trans-isomers.
References
- Vollhardt, K. P.C. & Shore, N. (2007). Organic Chemistry (5th Ed.). New York: W. H. Freeman (453-454)
- Vollhardt, K. P.C. & Shore, N (2007) Organic Chemistry: Study Guide and Solution Manuel (5th Ed.). New York: W.H Freeman (200-202)
Problems
1. Compound containing carbon-carbon double bonds are called:
- (a) Alkanes
- (b) Alkynes
- (c) Alkenes
- (d) Alcohols
2. Cis-alkenes exhibit a weak net dipole-dipole interactions:
- True
- False
3. Which has a lower melting poin
- (a) 5-Decene
- (b) 3-Octene
- (c) cis-2-Pentene
- (d) 1-Pentene
Contributors
- Trung Nguyen
Jim Clark (Chemguide.co.uk)

