Metal Oxides
- Page ID
- 37489
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- How does metal oxides protect a metal from further oxidation?
- How does oxidation rate progress on metal surfaces?
- How is iron erroded?
- What are the major iron oxides and their structures?
Defects in Metal Oxides
Metal oxides are very common commodities, widely applied, and have many different varieties. For example, zinc oxide sintered together with other metal oxide additives have been made into nonlinear resistors, which are called Varistors for surge suppressing function. The suppressing function has been applied for switching and for protection of random voltage protections. Iron oxide and other metal oxides are used in thermite reactions, and this has been applied in many ways, including welding in spaceship repairs. Iron oxides are also the raw material for all magnets and magnetic materials used for computer disks and recording tapes.
How does metal oxides protect a metal from further oxidation?
Metals are protected from further oxidation by forming a hard scale of oxides when it is being oxidized. Not all metal oxides form a scale. In general, when the oxide formed is not very dense, it is not under stress, and the oxide layer forms a scale. Usually, a mole of metal oxide should occupy more volume than a mole of the metal itself. If this is true, the oxide is not under stress, and a protective scale is formed. In general, if the volume of the metal oxide per mole of metal is greater than the molar volume of the metal, the oxide will form a protective scale.
On the other hand, if the oxide formed occupies a smaller volume than the volume occupied by the metal itself, the oxide layer will be under tension and at some point it will crack. Thus, the oxide offers no protection for further oxidation. The molar volume is easily calculated by dividing the molar mass by the density:
\[\text{molar volume} =\dfrac{\text{Molar mass}}{density} \nonumber \]
The densities of \(Mg\) and \(MgO\) are 1.74 and 3.58 g/mL respectively. Calculate their molar volumes.
Solution
The molar volumes are given below:
Molar volume of Mg = 24.31 (g/mol) / 1.74 (g/mL) = 14.0 mL / mol
Molar volume of MgO = (24.31+16.00) (g/mol) / 3.58 (g/mL) = 11.3 mL / mol
DISCUSSION
Since the molar volume of the oxide is less than that of the metal, the oxide does not form a protective scale.
The densities of \(Al\) and \(Al_2O_3\) are 2.702 and 3.965 g/mL respectively. Calculate their volumes per mole of Al.
Solution
The volumes per mole of La are given below:
Molar volume of Al = 26.98 (g/mol) / 2.702 (g/mL) = 9.985 mL / mol
Volume of Al2O3 = (26.98+24.00) (g/mol) / 3.965 (g/mL =12.86 mL / mol
DISCUSSION
It has been a well known fact that aluminum oxide forms a protective scale. These data confirm the fact, and now you have an explanation for corrosion. However, we should realize that sometimes the metal oxide does not form a protective layer even if the oxide is not under tension.
How does oxidation rate progress on metal surfaces?
A major technological concern of metals is the corrosion due to oxidation. The rate of oxidation is usually expressed in terms of depth of the oxidation layer. Several models have been proposed to express the thickness of the oxide layer y as a function of time t. In the following discussion, k is a rate constant.
The linear rule. When the oxide offers absolutely no protection, the progress of oxidation is a linear relationship with time. This has been called the rectilinear rate law by Swaddle.
\[dy = k dt \nonumber \]
or
\[y = k t \nonumber \]
When the oxide layer gives some protection, the parabolic law apply. This law is formulated with the consideration,
\[y dy = k dt \nonumber \]
or
\[y_2 = k' t + c \nonumber \]
with \(k' = 2 k\) and c is another constant
or
\[y = k" t 1/2 + c" \nonumber \]
with k" and c" other constants
Oxidation of copper has been shown to follow this rule. The thicker the oxide layer, the more protection the oxide offers in this case. When the oxide layer forms a protective layer, but large flakes crack and leas to faster oxidation as a result. Then, the rate is a combination of the linear rule and the parabolic law.
When the oxide forms a good protective layer, the logarithmic rate law and the inverse logarithmic rate law) have been applied. Some suggested formulas are:
\[ \dfrac{1}{y} = a \ln (k t +1) \nonumber \]
or
\[y = b ln (k t + 1) \nonumber \]
The a, b, and k are just some parameters to be determined by experimental methods. The properties of metals and its oxide play roles in the rate of corrosion. There is no set rate laws, and every case must be studied carefully. So far, we have hinted a few has models for exploration and analysis of corrosion problems.
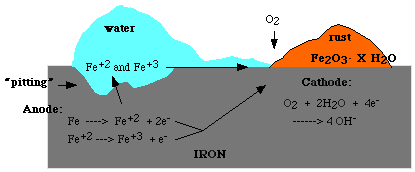
How is iron corroded?
The problem with iron as well as many other metals is that the oxide formed by oxidation does not firmly adhere to the surface of the metal and flakes off easily causing "pitting". Extensive pitting eventually causes structural weakness and disintegration of the metal. Most people consider the oxidation of iron results in the formation of a film of iron sesquioxide, which is a term given to red iron(III) oxide, Fe2O3, which is also called ferric oxide. In reality, the oxidation and corrosion of iron is a very complicated process.
The corrosion takes place due to oxidation and galvanic actions. The rust formed is generally represented by
\[Fe_2O_3 \cdot xH_2O \nonumber \]
where x is an unspecific amount of water. Depending on the amount of water, the oxides appear in various colors. The pH of water and electrolytes present in the water affect the rate of iron corrosion, because the presence of electrolytes increases the conductivity of the solution.
What are the major iron oxides and their structures?
As mentioned above, iron can be at oxidation state II or III in the form of Fe2+ or Fe3+ in its oxides. As a result, iron oxides tends to be somewhat non-stoichiometric.
The common iron oxides are:
ferrous oxide FeO
hematite a-Fe2O3
maghemite g-Fe2O3
magnetite Fe3O4
However, when water is involved, several oxyhydroxides are possible:
goethite a-FeOOH
akaganeite b-FeOOH
lepidocrocite g-FeOOH
There is no need to memorize all these minerals, but you get the idea of varieties of iron oxides and oxyhydroxides.
The basic structures of iron oxides and iron oxy hydroxides can be described as a close packing of oxygen (or hydroxide) with iron ions in the octahedral sites. The structures of goetite and hematite may be describe as an approximate hcp close packing of O2- or HO- ions with some of the octahedral sites occupied by iron ions. Thus, these two types of structures are usually designated as a phases.
The structures of lepidocrocite and maghemite may be described as an approximate ccp (fcc) packing of O2- or HO- ions with some of the octahedral sites occupied by iron ions. Thus, these two types of structures are usually designated as g phases. Some of the iron ions can be replaced by other metal ions, forming solid solutions in a process known as isomorphous substitution in terms of structural chemistry.
Questions
- What is the condition for a metal oxide to form a protective layer to prevent the metal from further oxidation?
- Which occupies a larger volume, 1 mole of MgO or 1 mole of Mg metal?
- Which occupies a larger volume, 0.5 mole of Al2O3 or 1 mole of Al metal?
- If the rate of errosion is expressed by the equation,
y = b ln (k t + 1)
What is the differential rate law?
Solutions
- Hint: The volume of the metal oxide per mole of metal is greater than the molar volume of the metal.
Skill -
Explain protection of metal from corrosion. - Hint: 1 mol of Mg metal.
- Hint: 0.5 mol of Al2O3
Skill -
Explain protection of metal from corrosion. - Hint: Work out dy/dt
Contributors and Attributions
Chung (Peter) Chieh (Professor Emeritus, Chemistry @ University of Waterloo)

