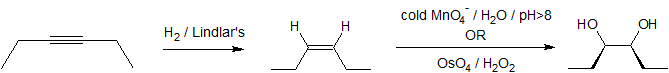
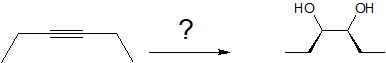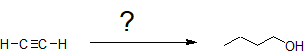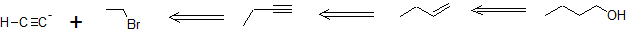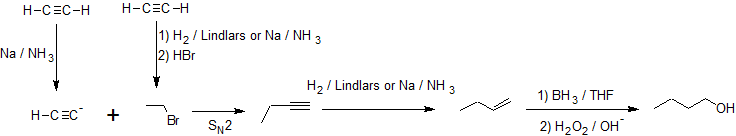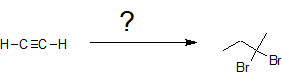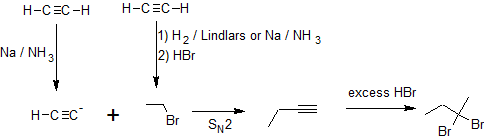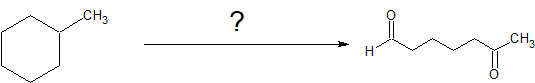A one or two step sequence of simple reactions is not that difficult to deduce. For example, the synthesis of meso-3,4-hexanediol from 3-hexyne can occur by more than one multi-step pathway.
One approach would be to reduce the alkyne to cis or trans-3-hexene before undertaking glycol formation. Permanaganate or osmium tetroxide hydroxylation of cis-3-hexene would form the desired meso isomer.
From trans-3-hexene, it would be necessary to first epoxidize the alkene with a peracid followed by ring opening with acidic or basic hydrolysis.
Longer multi-step syntheses require careful analysis and thought, since many options need to be considered. Like an expert chess player evaluating the long range pros and cons of potential moves, the chemist must appraise the potential success of various possible reaction paths, focusing on the scope and limitations constraining each of the individual reactions being employed. The skill is acquired by practice, experience, and often trial and error.
Thinking it Through with 3 Examples
The following three examples illustrate strategies for developing multi-step syntheses from the reactions studied in the first ten chapters of this text. It is helpful to systematically look for structural changes beginning with the carbon chain and brainstorm relevant functional group conversion reactions. Retro-synthesis is the approach of working backwards from the product to the starting material.
In the first example, we are asked to synthesize 1-butanol from acetylene.
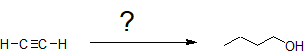
The carbon chain doubles in size indicating an acetylide SN2 reaction with an alkyl halide. Primary alcohol formation from an anti-Markovnikov alkene hydration reaction (hydroboration-oxidation) is more likely than a substitution reaction. Applying retro-synthesis, we work backwards from the alcohol to the alkene to the alkyne from an acetylide reaction that initially builds the carbon chain.
Retro-Synthesis
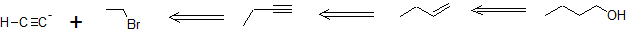
Working forwards, we specify the reagents needed for each transformation identified from the retro-synthesis. The ethylbromide must also be derived from acetylene so multiple reaction pathways are combined as shown below.
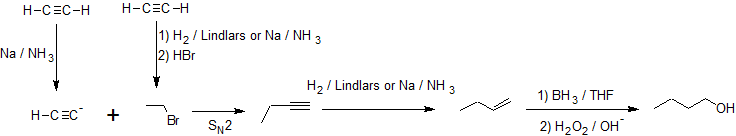
In the second example, we are asked to synthesize 1,2-dibromobutane from acetylene.
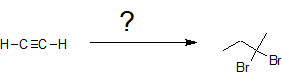
Once again there is an increase in the carbon chain length indicating an acetylide SN2 reaction with an alkyl halide similar to the first example. The hydrohalogenation can be subtle to discern because the hydrogen atoms are not shown in bond-line structures. Comparing the chemical formulas of 1-butyne with 1,2-dibromobutane, there is a difference of two H atoms and two Br atoms indicating hydrohalogenation and not halogenation. The addition of both bromine atoms to the same carbon atom also supports the idea that hydrohalogenation occurs on an alkyne and not an alkene. The formation of the geminal dihalide also indicates hydrohalogenation instead of halogenatioin because halogenation produces vicinal dihalides. With this insight, the retro-synthesis indicates the following series of chemical transformations.
Retro-Synthesis

Working forwards, we specify the reagents needed for each transformation.
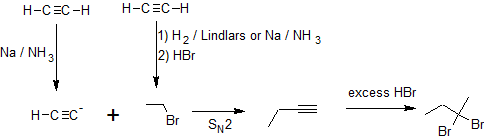
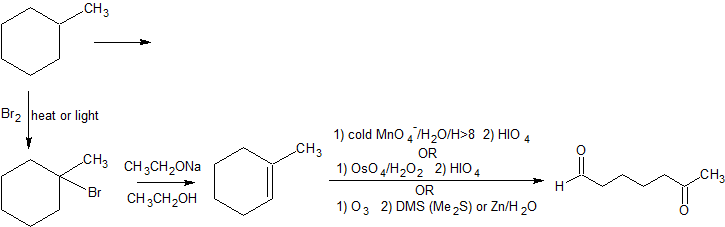
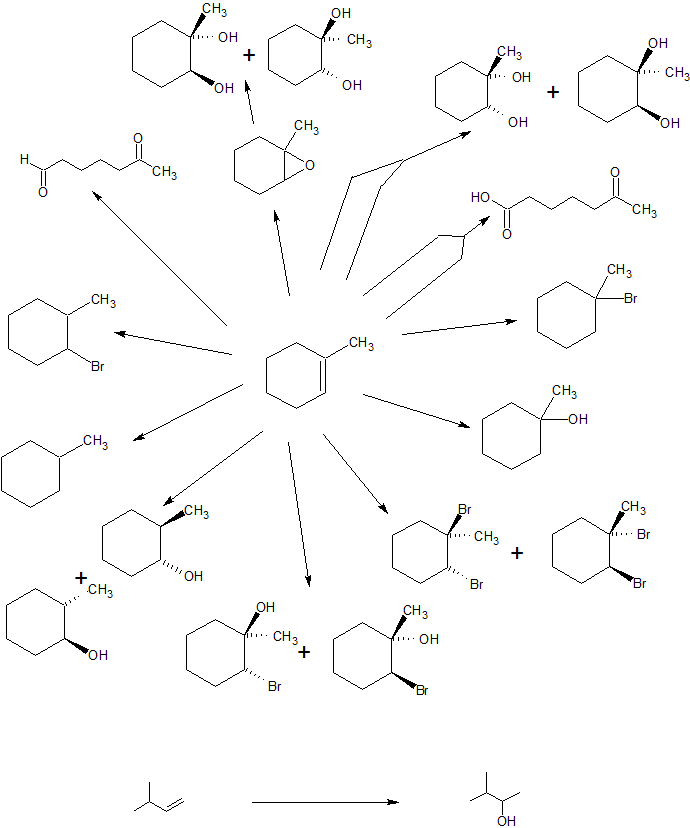
In the third example, we are asked to produce 6-oxoheptanal from methylcyclohexane.
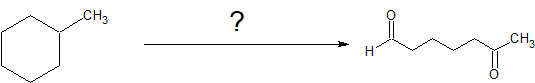
Counting the carbons, the starting material and product both contain seven carbon atoms and there is a cleavage reaction of an alkene under reductive conditions. One important missing aspect of this reaction is a good leaving group (LG). Alkanes are chemically quite boring. We can burn them as fuel or perform free-radical halogenation to create alkyl halides with excellent leaving groups. With these observations, the following retro-synthesis is reasonable.
Retro-Synthesis
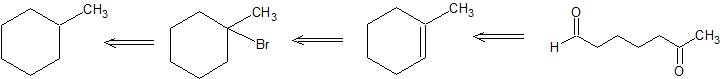
Working forwards, we specify the reagents needed for each reaction. For the initial free-radical halogenation of the alkane, we have the option of chlorine (Cl2) or bromine (Br2). Because methylcyclohexane has several different classifications of carbons, the selectivity of Br2 is more important than the faster reactivity of Cl2. An strong base with heat can be used for the second step to follow an E2 mechanism and form 1-methylcyclohexene. The aldhyde group on the final product indicates gentle oxidative cleavage by any of several reaction pathways. These reactions can be combined in to the following multi-step synthesis.