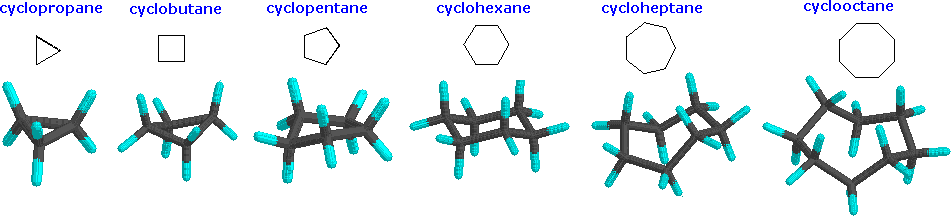
Cycloalkanes are very important in components of food, pharmaceutical drugs, and much more. However, to use cycloalkanes in such applications, we must know the effects, functions, properties, and structures of cycloalkanes. Cycloalkanes are alkanes that are in the form of a ring; hence, the prefix cyclo-. Stable cycloalkanes cannot be formed with carbon chains of just any length. Recall that in alkanes, carbon adopts the sp3 tetrahedral geometry in which the angles between bonds are 109.5°. For some cycloalkanes to form, the angle between bonds must deviate from this ideal angle, an effect known as angle strain. Additionally, some hydrogen atoms may come into closer proximity with each other than is desirable (become eclipsed), an effect called torsional strain. These destabilizing effects, angle strain and torsional strain are known together as ring strain. The smaller cycloalkanes, cyclopropane and cyclobutane, have particularly high ring strains because their bond angles deviate substantially from 109.5° and their hydrogens eclipse each other. Cyclopentane is a more stable molecule with a small amount of ring strain, while cyclohexane is able to adopt the perfect geometry of a cycloalkane in which all angles are the ideal 109.5° and no hydrogens are eclipsed; it has no ring strain at all. Cycloalkanes larger than cyclohexane have ring strain and are not commonly encountered in organic chemistry.
Ring Strain and the Structures of Cycloalkanes
There are many forms of cycloalkanes, such as cyclopropane, cyclobutane, cyclopentane, cyclohexane, among others. The process of naming cycloalkanes is the same as naming alkanes but the addition of the prefix cyclo- is required. Cyclobutane is in a form of a square, which is highly unfavorable and unstable (this will be explained soon). There are different drawings for cyclobutane, but they are equivalent to each other. Cyclobutane can reduce the ring string by puckering the square cyclobutane. Cyclopentane takes the shape of a pentagon and cyclohexane is in the shape of a hexagon.
There are two ways to draw cyclohexane because it can be in a hexagon shape or in a different conformational form called the chair conformation and the boat conformation.
- The chair conformation drawing is more favored than the boat because of the energy, the steric hindrance, and a new strain called the transannular strain.
- The boat conformation is not the favored conformation because it is less stable and has a steric repulsion between the two H's, shown with the pink curve. This is known as the transannular strain, which means that the strain results from steric crowding of two groups across a ring. The boat is less stable than the chair by 6.9 kcal/mol. The boat conformation, however, is flexible, and when we twist one of the C-C bonds, it reduces the transannular strain.
- When we twist the C-C bond in a boat, it becomes a twisted boat.
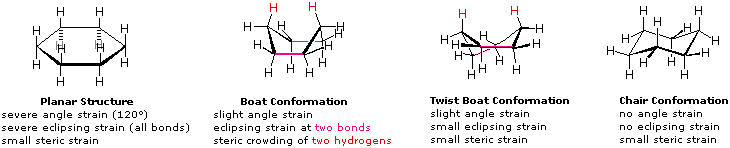
Some Conformations of Cyclohexane Rings. (William Reusch, MSU)
Although there are multiple ways to draw cyclohexane, the most stable and major conformer is the chair because is has a lower activation barrier from the energy diagram.
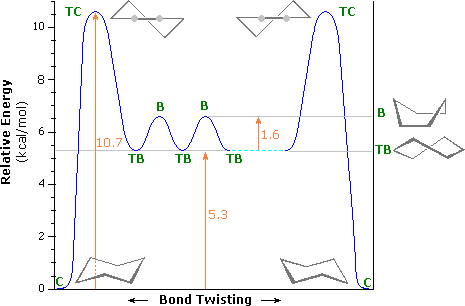
Conformational Energy Profile of Cyclohexane. (William Reusch, MSU).
The transition state structure is called a half chair. This energy diagram shows that the chair conformation is lower in energy; therefore, it is more stable. The chair conformation is more stable because it does not have any steric hindrance or steric repulsion between the hydrogen bonds. By drawing cyclohexane in a chair conformation, we can see how the H's are positioned. There are two positions for the H's in the chair conformation, which are in an axial or an equitorial formation.
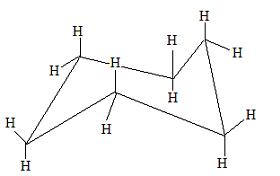
This is how a chair conformation looks, but you're probably wondering which H's are in the equitorial and axial form. Here are more pictures to help.
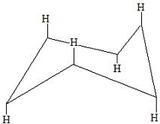
These are hydrogens in the axial form.
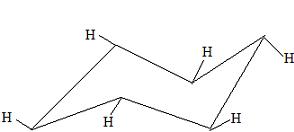
These hydrogens are in an equitorial form. Of these two positions of the H's, the equitorial form will be the most stable because the hydrogen atoms, or perhaps the other substituents, will not be touching each other. This is the best time to build a chair conformation in an equitorial and an axial form to demonstrate the stability of the equitorial form.
Ring Strain
Cycloalkanes tend to give off a very high and non-favorable energy, and the spatial orientation of the atoms is called the ring strain. When atoms are close together, their proximity is highly unfavorable and causes steric hindrance. The reason we do not want ring strain and steric hindrance is because heat will be released due to an increase in energy; therefore, a lot of that energy is stored in the bonds and molecules, causing the ring to be unstable and reactive. Another reason we try to avoid ring strain is because it will affect the structures and the conformational function of the smaller cycloalkanes. One way to determine the presence of ring strain is by its heat of combustion. By comparing the heat of combustion with the value measured for the straight chain molecule, we can determine the stability of the ring. There are two types of strain, which are eclipsing/torsional strain and bond angle strain. Bond angle strain causes a ring to have a poor overlap between the atoms, resulting in weak and reactive C-C bonds. An eclipsed spatial arrangement of the atoms on the cycloalkanes results in high energy.
With so many cycloalkanes, which ones have the highest ring strain and are very unlikely to stay in its current form? The figures below show cyclopropane, cyclobutane, and cyclopentane, respectively. Cyclopropane is one of the cycloalkanes that has an incredibly high and unfavorable energy, followed by cyclobutane as the next strained cycloalkane. Any ring that is small (with three to four carbons) has a significant amount of ring strain; cyclopropane and cyclobutane are in the category of small rings. A ring with five to seven carbons is considered to have minimal to zero strain, and typical examples are cyclopentane, cyclohexane, and cycloheptane. However, a ring with eight to twelve carbons is considered to have a moderate strain, and if a ring has beyond twelve carbons, it has minimal strain.
There are different types of ring strain:
- Transannular strain isdefined as the crowding of the two groups in a ring.
- Eclipsing strain, also known as torsional strain, is intramolecular strain due to the bonding interaction between two eclipsed atoms or groups.
- Bond angle strain is present when there is a poor overlap between the atoms. There must be an ideal bond angle to achieve the maximum bond strength and that will allow the overlapping of the atomic/hybrid orbitals.
Cyclohexane
Most of the time, cyclohexane adopts the fully staggered, ideal angle chair conformation. In the chair conformation, if any carbon-carbon bond were examined, it would be found to exist with its substituents in the staggered conformation and all bonds would be found to possess an angle of 109.5°.
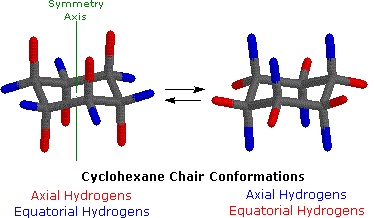
Cyclohexane in the chair conformation. (William Reusch, MSU).
In the chair conformation, hydrogen atoms are labeled according to their location. Those hydrogens which exist above or below the plane of the molecule (shown with red bonds above) are called axial. Those hydrogens which exist in the plane of the molecule (shown with blue bonds above) are called equatorial.
Although the chair conformation is the most stable conformation that cyclohexane can adopt, there is enough thermal energy for it to also pass through less favorable conformations before returning to a different chair conformation. When it does so, the axial and equatorial substituents change places. The passage of cyclohexane from one chair conformation to another, during which the axial substituents switch places with the equatorial substituents, is called a ring flip.
Methylcyclohexane
Methylcyclohexane is cyclohexane in which one hydrogen atom is replaced with a methyl group substituent. Methylcyclohexane can adopt two basic chair conformations: one in which the methyl group is axial, and one in which it is equatorial. Methylcyclohexane strongly prefers the equatorial conformation. In the axial conformation, the methyl group comes in close proximity to the axial hydrogens, an energetically unfavorable effect known as a 1,3-diaxial interaction (Figure 3). Thus, the equatorial conformation is preferred for the methyl group. In most cases, if the cyclohexane ring contains a substituent, the substituent will prefer the equatorial conformation.
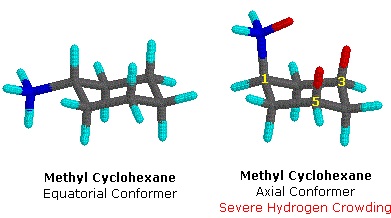 Methylcyclohexane in the chair conformation. (William Reusch, MSU).
Methylcyclohexane in the chair conformation. (William Reusch, MSU).
References
- Bachrach, Steven M. Computational Organic Chemistry. Wiley- Interscience. (2007).
- McMurray, John E. Organic Chemistry Sixth Edition. Brooks Cole. (2003).
- Vollhardt and Schore. Organic Chemistry Structure and Function Fifth Edition. New York. (2007).
Problems
- Trans- 1,2-dimethylcyclopropane is more stable than cis-1,2-dimethylcyclopropane. Why? Drawing a picture of the two will help your explanation.
- Out of all the cycloalkanes, which one has the most ring strain and which one is strain free? Explain.
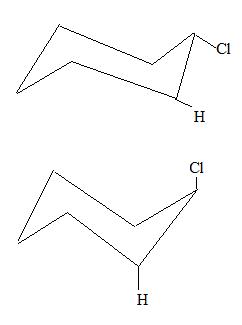
- Which of these chair conformations are the most stable and why?
3. 
- What does it mean when people say "increase in heat leads to increase in energy" and how does that statement relate to ring strains?
- Why is that the bigger rings have lesser strains compared to smaller rings?
Answers
- The cis isomer suffers from steric hindrance and has a larger heat of combustion.
- Cyclopropane- ring strain. Cyclohexane chair conformation- ring strain free.
- Top one is more stable because it is in an equitorial conformation. When assembling it with the OChem Molecular Structure Tool Kit, equitorial formation is more spread out.
- When there is an increase in heat there will be an increase of energy released therefore there will be a lot of energy stored in the bond and molecule making it unstable.
- Smaller rings are more compacts, which leads to steric hindrance and the angles for these smaller rings are harder to get ends to meet. Bigger rings tend to have more space and that the atoms attached to the ring won't be touching each other as much as atoms attached to the smaller ring.









 Methylcyclohexane in the chair conformation. (William Reusch, MSU).
Methylcyclohexane in the chair conformation. (William Reusch, MSU).
