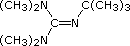Boiling Point and Water Solubility
It is instructive to compare the boiling points and water solubility of amines with those of corresponding alcohols and ethers. The dominant factor here is hydrogen bonding, and the first table below documents the powerful intermolecular attraction that results from -O-H---O- hydrogen bonding in alcohols (light blue columns). Corresponding -N-H---N- hydrogen bonding is weaker, as the lower boiling points of similarly sized amines (light green columns) demonstrate. Alkanes provide reference compounds in which hydrogen bonding is not possible, and the increase in boiling point for equivalent 1º-amines is roughly half the increase observed for equivalent alcohols.
| Compound |
CH3CH3 |
CH3OH |
CH3NH2 |
CH3CH2CH3 |
CH3CH2OH |
CH3CH2NH2 |
| Mol.Wt. |
30 |
32 |
31 |
44 |
46 |
45 |
Boiling
Point ºC |
-88.6º |
65º |
-6.0º |
-42º |
78.5º |
16.6º |
The second table illustrates differences associated with isomeric 1º, 2º & 3º-amines, as well as the influence of chain branching. Since 1º-amines have two hydrogens available for hydrogen bonding, we expect them to have higher boiling points than isomeric 2º-amines, which in turn should boil higher than isomeric 3º-amines (no hydrogen bonding). Indeed, 3º-amines have boiling points similar to equivalent sized ethers; and in all but the smallest compounds, corresponding ethers, 3º-amines and alkanes have similar boiling points. In the examples shown here, it is further demonstrated that chain branching reduces boiling points by 10 to 15 ºC.
| Compound |
CH3(CH2)2CH3 |
CH3(CH2)2OH |
CH3(CH2)2NH2 |
CH3CH2NHCH3 |
(CH3)3CH |
(CH3)2CHOH |
(CH3)2CHNH2 |
(CH3)3N |
| Mol.Wt. |
58 |
60 |
59 |
59 |
58 |
60 |
59 |
59 |
Boiling
Point ºC |
-0.5º |
97º |
48º |
37º |
-12º |
82º |
34º |
3º |
The water solubility of 1º and 2º-amines is similar to that of comparable alcohols. As expected, the water solubility of 3º-amines and ethers is also similar. These comparisons, however, are valid only for pure compounds in neutral water. The basicity of amines (next section) allows them to be dissolved in dilute mineral acid solutions, and this property facilitates their separation from neutral compounds such as alcohols and hydrocarbons by partitioning between the phases of non-miscible solvents.
Basicity of Amines
A review of basic acid-base concepts should be helpful to the following discussion. Like ammonia, most amines are Brønsted and Lewis bases, but their base strength can be changed enormously by substituents. It is common to compare basicity's quantitatively by using the pKa's of their conjugate acids rather than their pKb's. Since pKa + pKb = 14, the higher the pKa the stronger the base, in contrast to the usual inverse relationship of pKa with acidity. Most simple alkyl amines have pKa's in the range 9.5 to 11.0, and their water solutions are basic (have a pH of 11 to 12, depending on concentration). The first four compounds in the following table, including ammonia, fall into that category.
The last five compounds (colored cells) are significantly weaker bases as a consequence of three factors. The first of these is the hybridization of the nitrogen. In pyridine the nitrogen is sp2 hybridized, and in nitriles (last entry) an sp hybrid nitrogen is part of the triple bond. In each of these compounds (shaded red) the non-bonding electron pair is localized on the nitrogen atom, but increasing s-character brings it closer to the nitrogen nucleus, reducing its tendency to bond to a proton.
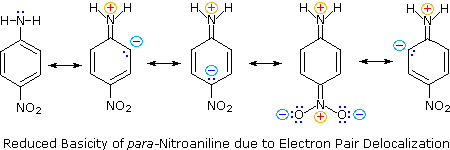
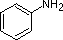
Secondly, aniline and p-nitroaniline (first two green shaded structures) are weaker bases due to delocalization of the nitrogen non-bonding electron pair into the aromatic ring (and the nitro substituent). This is the same delocalization that results in activation of a benzene ring toward electrophilic substitution. The following resonance equations, which are similar to those used to explain the enhanced acidity of ortho and para-nitrophenols illustrate electron pair delocalization in p-nitroaniline. Indeed, aniline is a weaker base than cyclohexyl amine by roughly a million fold, the same factor by which phenol is a stronger acid than cyclohexanol. This electron pair delocalization is accompanied by a degree of rehybridization of the amino nitrogen atom, but the electron pair delocalization is probably the major factor in the reduced basicity of these compounds. A similar electron pair delocalization is responsible for the very low basicity (and nucleophilic reactivity) of amide nitrogen atoms (last green shaded structure). This feature was instrumental in moderating the influence of amine substituents on aromatic ring substitution, and will be discussed further in the section devoted to carboxylic acid derivatives.
Conjugated amine groups influence the basicity of an existing amine. Although 4-dimethylaminopyridine (DMAP) might appear to be a base similar in strength to pyridine or N,N-dimethylaniline, it is actually more than ten thousand times stronger, thanks to charge delocalization in its conjugate acid. The structure in the gray box shows the locations over which positive charge (colored red) is delocalized in the conjugate acid. This compound is often used as a catalyst for acyl transfer reactions.
Finally, the very low basicity of pyrrole (shaded blue) reflects the exceptional delocalization of the nitrogen electron pair associated with its incorporation in an aromatic ring. Indole (pKa = -2) and imidazole (pKa = 7.0), see above, also have similar heterocyclic aromatic rings. Imidazole is over a million times more basic than pyrrole because the sp2 nitrogen that is part of one double bond is structurally similar to pyridine, and has a comparable basicity.
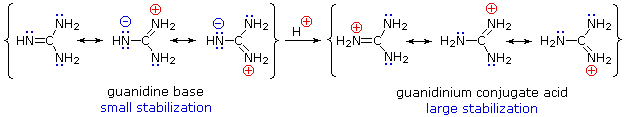
Although resonance delocalization generally reduces the basicity of amines, a dramatic example of the reverse effect is found in the compound guanidine (pKa = 13.6). Here, as shown below, resonance stabilization of the base is small, due to charge separation, while the conjugate acid is stabilized strongly by charge delocalization. Consequently, aqueous solutions of guanidine are nearly as basic as are solutions of sodium hydroxide.
The relationship of amine basicity to the acidity of the corresponding conjugate acids may be summarized in a fashion analogous to that noted earlier for acids:
Strong bases have weak conjugate acids, and weak bases have strong conjugate acids.
Acidity of Amines
We normally think of amines as bases, but it must be remembered that 1º and 2º-amines are also very weak acids (ammonia has a pKa = 34). In this respect it should be noted that pKa is being used as a measure of the acidity of the amine itself rather than its conjugate acid, as in the previous section. For ammonia this is expressed by the following hypothetical equation:
NH3 + H2O ____> NH2(–) + H2O-H(+)
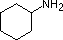
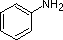
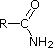
The same factors that decreased the basicity of amines increase their acidity. This is illustrated by the following examples, which are shown in order of increasing acidity. It should be noted that the first four examples have the same order and degree of increased acidity as they exhibited decreased basicity in the previous table. The first compound is a typical 2º-amine, and the three next to it are characterized by varying degrees of nitrogen electron pair delocalization. The last two compounds (shaded blue) show the influence of adjacent sulfonyl and carbonyl groups on N-H acidity. From previous discussion it should be clear that the basicity of these nitrogens is correspondingly reduced.
| Compound |
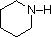 |
 |
 |
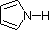 |
C6H5SO2NH2 |
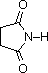 |
| pKa |
33 |
27 |
19 |
15 |
10 |
9.6 |
The acids shown here may be converted to their conjugate bases by reaction with bases derived from weaker acids (stronger bases). Three examples of such reactions are shown below, with the acidic hydrogen colored red in each case. For complete conversion to the conjugate base, as shown, a reagent base roughly a million times stronger is required.
C6H5SO2NH2 + KOH  C6H5SO2NH(–) K(+) + H2O C6H5SO2NH(–) K(+) + H2O |
a sulfonamide base |
(CH3)3COH + NaH  (CH3)3CO(–) Na(+) + H2 (CH3)3CO(–) Na(+) + H2 |
an alkoxide base |
(C2H5)2NH + C4H9Li  (C2H5)2N(–) Li(+) + C4H10 (C2H5)2N(–) Li(+) + C4H10 |
an amide base |
Important Reagent Bases
The significance of all these acid-base relationships to practical organic chemistry lies in the need for organic bases of varying strength, as reagents tailored to the requirements of specific reactions. The common base sodium hydroxide is not soluble in many organic solvents, and is therefore not widely used as a reagent in organic reactions. Most base reagents are alkoxide salts, amines or amide salts. Since alcohols are much stronger acids than amines, their conjugate bases are weaker than amide bases, and fill the gap in base strength between amines and amide salts. In the following table, pKa again refers to the conjugate acid of the base drawn above it.
| Base Name |
Pyridine |
Triethyl
Amine |
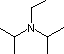
Hünig's Base |
Barton's
Base |
Potassium
t-Butoxide |
Sodium HMDS |
LDA |
| Formula |
 |
(C2H5)3N |
 |
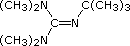 |
(CH3)3CO(–) K(+) |
[(CH3)3Si]2N(–) Na(+) |
[(CH3)2CH]2N(–) Li(+) |
| pKa |
5.3 |
10.7 |
11.4 |
14 |
19 |
26 |
35.7 |
Pyridine is commonly used as an acid scavenger in reactions that produce mineral acid co-products. Its basicity and nucleophilicity may be modified by steric hindrance, as in the case of 2,6-dimethylpyridine (pKa=6.7), or resonance stabilization, as in the case of 4-dimethylaminopyridine (pKa=9.7). Hünig's base is relatively non-nucleophilic (due to steric hindrance), and like DBU is often used as the base in E2 elimination reactions conducted in non-polar solvents. Barton's base is a strong, poorly-nucleophilic, neutral base that serves in cases where electrophilic substitution of DBU or other amine bases is a problem. The alkoxides are stronger bases that are often used in the corresponding alcohol as solvent, or for greater reactivity in DMSO. Finally, the two amide bases see widespread use in generating enolate bases from carbonyl compounds and other weak carbon acids.
Nonionic Superbases
An interesting group of neutral, highly basic compounds of nitrogen and phosphorus have been prepared, and are referred to as superbases. To see examples of these compounds Click Here.


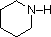
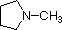


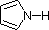


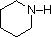


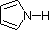

 C6H5SO2NH(–) K(+) + H2O
C6H5SO2NH(–) K(+) + H2O (CH3)3CO(–) Na(+) + H2
(CH3)3CO(–) Na(+) + H2  (C2H5)2N(–) Li(+) + C4H10
(C2H5)2N(–) Li(+) + C4H10