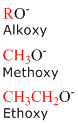Alkanes with unbranched carbon chains are simply named by the number of carbons in the chain. The first four members of the series (in terms of number of carbon atoms) are named as follows:
Number of Hydrogen to Carbons
This equation describes the relationship between the number of hydrogen and carbon atoms in alkanes:
- H = 2C + 2
where "C" and "H" are used to represent the number of carbon and hydrogen atoms present in one molecule. If C = 2, then H = 6.
Many textbooks put this in the following format:
- CnH2n+2
where "Cn" and "H2n+2" represent the number of carbon and hydrogen atoms present in one molecule. If Cn = 3, then H2n+2 = 2(3) + 2 = 8. (For this formula look to the "n" for the number, the "C" and the "H" letters themselves do not change.)
Progressively longer hydrocarbon chains can be made and are named systematically, depending on the number of carbons in the longest chain.
The following table contains the systematic names for the first twenty straight chain alkanes. It will be important to familiarize yourself with these names because they will be the basis for naming many other organic molecules throughout your course of study.
Drawing Hydrocarbons
Recall that when carbon makes four bonds, it adopts the tetrahedral geometry. In the tetrahedral geometry, only two bonds can occupy a plane simultaneously. The other two bonds point in back or in front of this plane. In order to represent the tetrahedral geometry in two dimensions, solid wedges are used to represent bonds pointing out of the plane of the drawing toward the viewer, and dashed wedges are used to represent bonds pointing out of the plane of the drawing away from the viewer. Consider the following representation of the molecule methane:
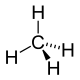
Figure 1: Two dimensional representation of methane
In the above drawing, the two hydrogens connected by solid lines, as well as the carbon in the center of the molecule, exist in a plane (specifically, the plane of the computer monitor / piece of paper, etc.). The hydrogen connected by a solid wedge points out of this plane toward the viewer, and the hydrogen connected by the dashed wedge points behind this plane and away from the viewer.
In drawing hydrocarbons, it can be time-consuming to write out each atom and bond individually. In organic chemistry, hydrocarbons can be represented in a shorthand notation called a skeletal structure. In a skeletal structure, only the bonds between carbon atoms are represented. Individual carbon and hydrogen atoms are not drawn, and bonds to hydrogen are not drawn. In the case that the molecule contains just single bonds (sp3 bonds), these bonds are drawn in a "zig-zag" fashion. This is because in the tetrahedral geometry all bonds point as far away from each other as possible, and the structure is not linear. Consider the following representations of the molecule propane:
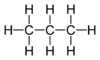
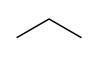
Figure 2: Full structure of propane and skeletal structure of propane
Only the bonds between carbons have been drawn, and these have been drawn in a "zig-zag" manner. Note that there is no representation of hydrogens in a skeletal structure. Since, in the absence of double or triple bonds, carbon makes four bonds total, the presence of hydrogens is implicit. Whenever an insufficient number of bonds to a carbon atom are specified in the structure, it is assumed that the rest of the bonds are made to hydrogens. For example, if the carbon atom makes only one explicit bond, there are three hydrogens implicitly attached to it. If it makes two explicit bonds, there are two hydrogens implicitly attached, etc. Note also that two lines are sufficient to represent three carbon atoms. It is the bonds only that are being drawn out, and it is understood that there are carbon atoms (with three hydrogens attached!) at the terminal ends of the structure.
Alkyl Groups
Alkanes can be described by the general formula CnH2n+2. An alkyl group is formed by removing one hydrogen from the alkane chain and is described by the formula CnH2n+1. The removal of this hydrogen results in a stem change from -ane to -yl. Take a look at the following examples.
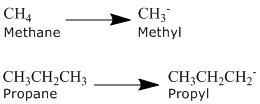
The same concept can be applied to any of the straight chain alkane names provided in the table above.
| Name |
Molecular Formula |
Condensed Structural Formula |
| Methane |
CH4 |
CH4 |
| Ethane |
C2H6 |
CH3CH3 |
| Propane |
C3H8 |
CH3CH2CH3 |
| Butane |
C4H10 |
CH3(CH2)2CH3 |
| Pentane |
C5H12 |
CH3(CH2)3CH3 |
| Hexane |
C6H14 |
CH3(CH2)4CH3 |
| Heptane |
C7H16 |
CH3(CH2)5CH3 |
| Octane |
C8H18 |
CH3(CH2)6CH3 |
| Nonane |
C9H20 |
CH3(CH2)7CH3 |
| Decane |
C10H22 |
CH3(CH2)8CH3 |
| Undecane |
C11H24 |
CH3(CH2)9CH3 |
| Dodecane |
C12H26 |
CH3(CH2)10CH3 |
| Tridecane |
C13H28 |
CH3(CH2)11CH3 |
| Tetradecane |
C14H30 |
CH3(CH2)12CH3 |
| Pentadecane |
C15H32 |
CH3(CH2)13CH3 |
| Hexadecane |
C16H34 |
CH3(CH2)14CH3 |
| Heptadecane |
C17H36 |
CH3(CH2)15CH3 |
| Octadecane |
C18H38 |
CH3(CH2)16CH3 |
| Nonadecane |
C19H40 |
CH3(CH2)17CH3 |
| Eicosane |
C20H42 |
CH3(CH2)18CH3 |
Using Common Names with Branched Alkanes
Certain branched alkanes have common names that are still widely used today. These common names make use of prefixes, such as iso-, sec-, tert-, and neo-. The prefix iso-, which stands for isomer, is commonly given to 2-methyl alkanes. In other words, if there is methyl group located on the second carbon of a carbon chain, we can use the prefix iso-. The prefix will be placed in front of the alkane name that indicates the total number of carbons. Examples:
- isopentane which is the same as 2-methylbutane
- isobutane which is the same as 2-methylpropane
To assign the prefixes sec-, which stands for secondary, and tert-, for tertiary, it is important that we first learn how to classify carbon molecules. If a carbon is attached to only one other carbon, it is called a primary carbon. If a carbon is attached to two other carbons, it is called a seconday carbon. A tertiary carbon is attached to three other carbons and last, a quaternary carbon is attached to four other carbons. Examples:
- 4-sec-butylheptane (30g)
- 4-tert-butyl-5-isopropylhexane (30d); if using this example, may want to move sec/tert after iso disc
The prefix neo- refers to a substituent whose second-to-last carbon of the chain is trisubstituted (has three methyl groups attached to it). A neo-pentyl has five carbons total. Examples:
Alkoxy Groups
Alkoxides consist of an organic group bonded to a negatively charged oxygen atom. In the general form, alkoxides are written as RO-, where R represents the organic substituent. Similar to the alkyl groups above, the concept of naming alkoxides can be applied to any of the straight chain alkanes provided in the table above.
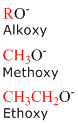
Three Principles of Naming
- Choose the longest, most substituted carbon chain containing a functional group.
- A carbon bonded to a functional group must have the lowest possible carbon number. If there are no functional groups, then any substitute present must have the lowest possible number.
- Take the alphabetical order into consideration; that is, after applying the first two rules given above, make sure that your substitutes and/or functional groups are written in alphabetical order.
Example 1
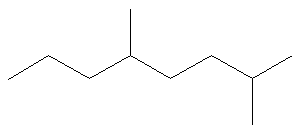
What is the name of the following molecule?
Solution
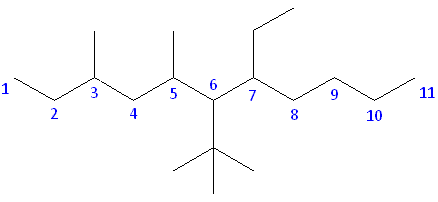
Rule #1: Choose the longest, most substituted carbon chain containing a functional group. This example does not contain any functional groups, so we only need to be concerned with choosing the longest, most substituted carbon chain. The longest carbon chain has been highlighted in red and consists of eight carbons.
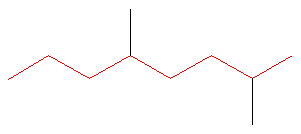
Rule #2: Carbons bonded to a functional group must have the lowest possible carbon number. If there are no functional groups, then any substitute present must have the lowest possible number. Because this example does not contain any functional groups, we only need to be concerned with the two substitutes present, that is, the two methyl groups. If we begin numbering the chain from the left, the methyls would be assigned the numbers 4 and 7, respectively. If we begin numbering the chain from the right, the methyls would be assigned the numbers 2 and 5. Therefore, to satisfy the second rule, numbering begins on the right side of the carbon chain as shown below. This gives the methyl groups the lowest possible numbering.
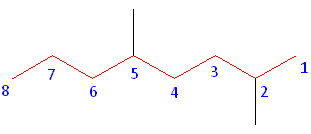
Rule 3: In this example, there is no need to utilize the third rule. Because the two substitutes are identical, neither takes alphabetical precedence with respect to numbering the carbons. This concept will become clearer in the following examples.
Example 2
What is the name of the following molecule?

Solution
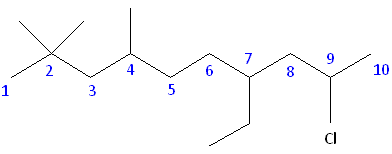
Rule #1: Choose the longest, most substituted carbon chain containing a functional group. This example contains two functional groups, bromine and chlorine. The longest carbon chain has been highlighted in red and consists of seven carbons.
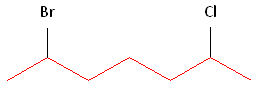
Rule #2: Carbons bonded to a functional group must have the lowest possible carbon number. If there are no functional groups, then any substitute present must have the lowest possible number. In this example, numbering the chain from the left or the right would satisfy this rule. If we number the chain from the left, bromine and chlorine would be assigned the second and sixth carbon positions, respectively. If we number the chain from the right, chlorine would be assigned the second position and bromine would be assigned the sixth position. In other words, whether we choose to number from the left or right, the functional groups occupy the second and sixth positions in the chain. To select the correct numbering scheme, we need to utilize the third rule.
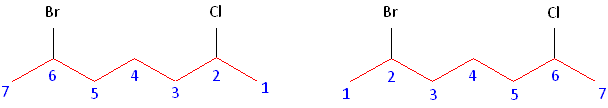
Rule #3: After applying the first two rules, take the alphabetical order into consideration. Alphabetically, bromine comes before chlorine. Therefore, bromine is assigned the second carbon position, and chlorine is assigned the sixth carbon position.
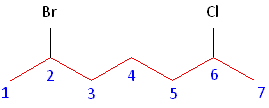
Example 3
What is the name of the follow molecule?
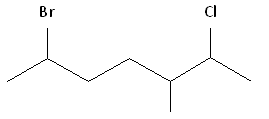
Solution
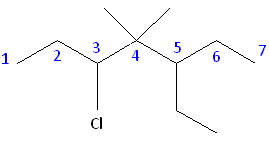
Rule #1: Choose the longest, most substituted carbon chain containing a functional group. This example contains two functional groups, bromine and chlorine, and one substitute, the methyl group. The longest carbon chain has been highlighted in red and consists of seven carbons.
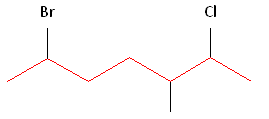
Rule #2: Carbons bonded to a functional group must have the lowest possible carbon number. After taking functional groups into consideration, any substitutes present must have the lowest possible carbon number. This particular example illustrates the point of difference principle. If we number the chain from the left, bromine, the methyl group and chlorine would occupy the second, fifth and sixth positions, respectively. This concept is illustrated in the second drawing below. If we number the chain from the right, chlorine, the methyl group and bromine would occupy the second, third and sixth positions, respectively, which is illustrated in the first drawing below. The position of the methyl, therefore, becomes a point of difference. In the first drawing, the methyl occupies the third position. In the second drawing, the methyl occupies the fifth position. To satisfy the second rule, we want to choose the numbering scheme that provides the lowest possible numbering of this substitute. Therefore, the first of the two carbon chains shown below is correct.
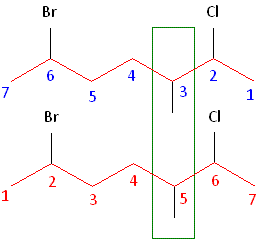
Therefore, the first numbering scheme is the appropriate one to use.
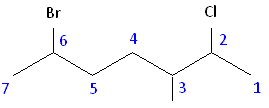
Once you have determined the correct numbering of the carbons, it is often useful to make a list, including the functional groups, substitutes, and the name of the parent chain.
Rule #3: After applying the first two rules, take the alphabetical order into consideration. Alphabetically, bromine comes before chlorine. Therefore, bromine is assigned the second carbon position, and chlorine is assigned the sixth carbon position.
Parent chain: heptane 2-Chloro 3-Methyl 6-Bromo
6-bromo-2-chloro-3-methylheptane
Problems
What is the name of the follow molecules?