4.4: Determining the Limiting Reactant
- Page ID
- 19953
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)The Learning Objective of this Module is to understand the concept of limiting reactants.
Consider a nonchemical example. Assume you have invited some friends for dinner and want to bake brownies for dessert. You find two boxes of brownie mix in your pantry and see that each package requires two eggs. The balanced equation for brownie preparation is thus
\[ 1 \, box \, mix + 2 \, eggs \rightarrow 1 \, batch \, brownies \tag{3.21}\]
If you have a dozen eggs, which ingredient will determine the number of batches of brownies that you can prepare? Because each box of brownie mix requires two eggs and you have two boxes, you need four eggs. Twelve eggs is eight more eggs than you need. Although the ratio of eggs to boxes in is 2:1, the ratio in your possession is 6:1. Hence the eggs are the ingredient (reactant) present in excess, and the brownie mix is the limiting reactant. Even if you had a refrigerator full of eggs, you could make only two batches of brownies.
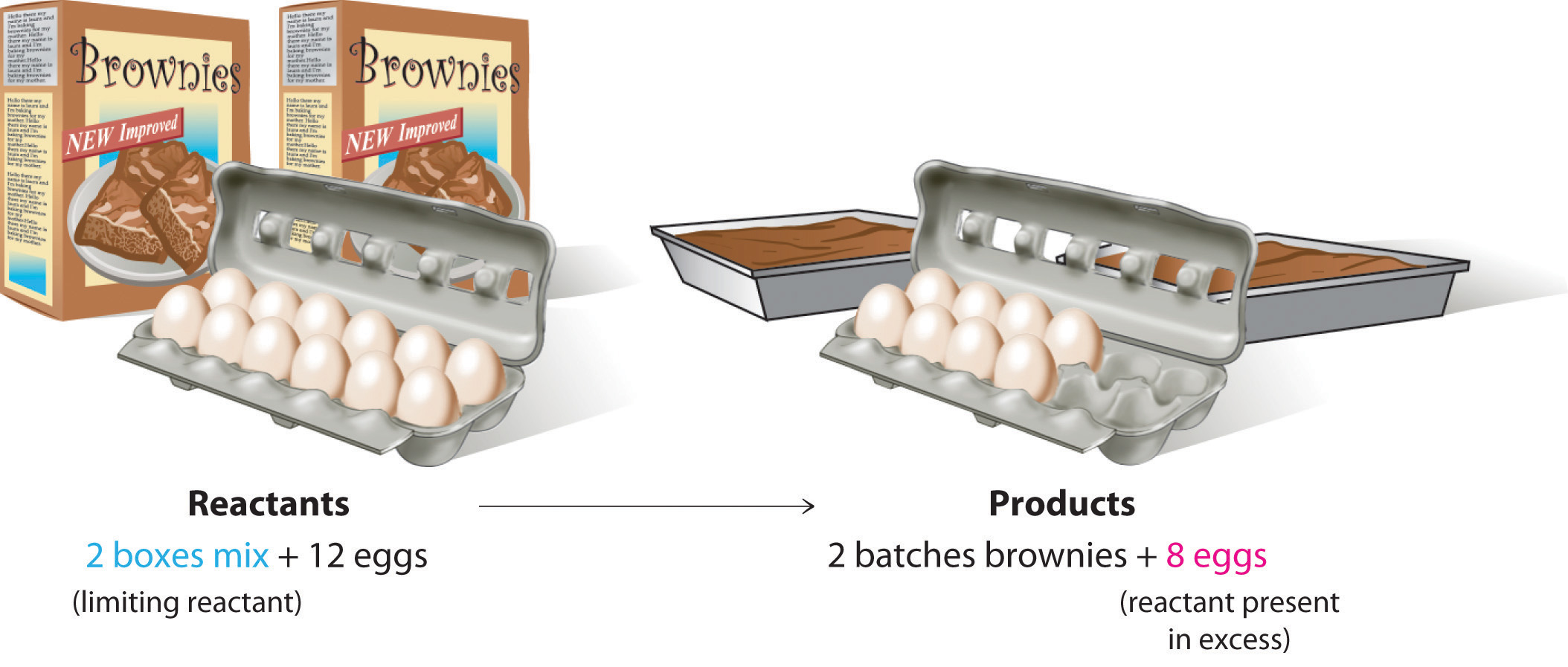
Figure 3.12 The Concept of a Limiting Reactant in the Preparation of Brownies
Now consider a chemical example of a limiting reactant: the production of pure titanium. This metal is fairly light (45% lighter than steel and only 60% heavier than aluminum) and has great mechanical strength (as strong as steel and twice as strong as aluminum). Because it is also highly resistant to corrosion and can withstand extreme temperatures, titanium has many applications in the aerospace industry. Titanium is also used in medical implants and portable computer housings because it is light and resistant to corrosion. Although titanium is the ninth most common element in Earth’s crust, it is relatively difficult to extract from its ores. In the first step of the extraction process, titanium-containing oxide minerals react with solid carbon and chlorine gas to form titanium tetrachloride (TiCl4) and carbon dioxide. Titanium tetrachloride is then converted to metallic titanium by reaction with magnesium metal at high temperature:
\[ TiCl_4 (g) + 2 \, Mg (l) \rightarrow Ti (s) + 2 \, MgCl_2 (l) \tag{3.22}\]
Because titanium ores, carbon, and chlorine are all rather inexpensive, the high price of titanium (about $100 per kilogram) is largely due to the high cost of magnesium metal. Under these circumstances, magnesium metal is the limiting reactant in the production of metallic titanium.
Medical use of titanium. Here is an example of its successful use in joint replacement implants. An A-P X-ray of a pelvis showing a total hip joint replacement. The right hip joint (on the left in the photograph) has been replaced. A metal prostheses is cemented in the top of the right femur and the head of the femur has been replaced by the rounded head of the prosthesis. Figure curtesty of NIH (NIADDK) 9AO4 (Connie Raab)
With 1.00 kg of titanium tetrachloride and 200 g of magnesium metal, how much titanium metal can be produced according to the equation above? Solving this type of problem requires that you carry out the following steps:
- Determine the number of moles of each reactant.
- Compare the mole ratio of the reactants with the ratio in the balanced chemical equation to determine which reactant is limiting.
- Calculate the number of moles of product that can be obtained from the limiting reactant.
- Convert the number of moles of product to mass of product.
1. To determine the number of moles of reactants present, calculate or look up their molar masses: 189.679 g/mol for titanium tetrachloride and 24.305 g/mol for magnesium. The number of moles of each is calculated as follows:
\[ moles \, TiCl_4 = {mass \, TiCl_4 \over molar \, mass \, TiCl_4} \]
\[ = 1000 \, g \, TiCl_4 \times {1 \, mol \, TiCl_4 \over 189.679 \, g \, TiCl_4} = 5.272 \, mol \, TiCl_4 \]
\[ moles \, Mg = {mass \, Mg \over molar \, mass \, Mg}\]
\[ = 200 \, g \, Mg \times {1 \, mol \, Mg \over 24.305 \, g \, Mg } = 8.23 \, mol \, Mg \]
2. There are more moles of magnesium than of titanium tetrachloride, but the ratio is only the following:
\[ {mol \, Mg \over mol \, TiCl_4} = {8.23 \, mol \over 5.272 \, mol } = 1.56 \]
Because the ratio of the coefficients in the balanced chemical equation is,
\[{ 2 \, mol \, Mg \over 1 \, mol \, TiCl_4} = 2 \]
there is not have enough magnesium to react with all the titanium tetrachloride. If this point is not clear from the mole ratio, calculate the number of moles of one reactant that is required for complete reaction of the other reactant. For example, there are 8.23 mol of Mg, so (8.23 ÷ 2) = 4.12 mol of TiCl4 are required for complete reaction. Because there are 5.272 mol of TiCl4, titanium tetrachloride is present in excess. Conversely, 5.272 mol of TiCl4 requires 2 × 5.272 = 10.54 mol of Mg, but there are only 8.23 mol. Therefore, magnesium is the limiting reactant.
3. Because magnesium is the limiting reactant, the number of moles of magnesium determines the number of moles of titanium that can be formed:
\[ moles \, Ti = 8.23 \, mol \, Mg = {1 \, mol \, Ti \over 2 \, mol \, Mg} = 4.12 \, mol \, Ti \]
Thus only 4.12 mol of Ti can be formed.
4. To calculate the mass of titanium metal that can obtain, multiply the number of moles of titanium by the molar mass of titanium (47.867 g/mol):
\[ moles \, Ti = mass \, Ti \times molar \, mass \, Ti = 4.12 \, mol \, Ti \times {47.867 \, g \, Ti \over 1 \, mol \, Ti} = 197 \, g \, Ti \]
Here is a simple and reliable way to identify the limiting reactant in any problem of this sort:
- Calculate the number of moles of each reactant present: 5.272 mol of TiCl4 and 8.23 mol of Mg.
- Divide the actual number of moles of each reactant by its stoichiometric coefficient in the balanced chemical equation: \[ TiCl_4 : { 5.272 \, mol \, (actual) \over 1 \, mol \, (stoich)} = 5.272 \, \, \, \, Mg: {8.23 \, mol \, (actual) \over 2 \, mol \, (stoich)} = 4.12 \]
- The reactant with the smallest mole ratio is limiting. Magnesium, with a calculated stoichiometric mole ratio of 4.12, is the limiting reactant.
Density is the mass per unit volume of a substance. If we are given the density of a substance, we can use it in stoichiometric calculations involving liquid reactants and/or products, as Example 12 demonstrates.
| Example 12 |
|---|
| Ethyl acetate (CH3CO2C2H5) is the solvent in many fingernail polish removers and is used to decaffeinate coffee beans and tea leaves. It is prepared by reacting ethanol (C2H5OH) with acetic acid (CH3CO2H); the other product is water. A small amount of sulfuric acid is used to accelerate the reaction, but the sulfuric acid is not consumed and does not appear in the balanced chemical equation. Given 10.0 mL each of acetic acid and ethanol, how many grams of ethyl acetate can be prepared from this reaction? The densities of acetic acid and ethanol are 1.0492 g/mL and 0.7893 g/mL, respectively.  Given: reactants, products, and volumes and densities of reactants Asked for: mass of product Strategy:
Solution: A Always begin by writing the balanced chemical equation for the reaction: \[ C_2H_5OH (l) + CH_3CO_2H (aq) \rightarrow CH_3CO_2C_2H_5 (aq) + H_2O (l) \] B We need to calculate the number of moles of ethanol and acetic acid that are present in 10.0 mL of each. Recall from that the density of a substance is the mass divided by the volume: \[ density = {mass \over volume } \] Rearranging this expression gives mass = (density)(volume). We can replace mass by the product of the density and the volume to calculate the number of moles of each substance in 10.0 mL (remember, 1 mL = 1 cm3): \[ moles \, C_2H_5OH = { mass \, C_2H_5OH \over molar \, mass \, C_2H_5OH } \] \[ = {volume \, C_2H_5OH \times density \, C_2H_5OH \over molar \, mass \, C_2H_5OH}\] \[ = 10.0 \, ml \, C_2H_5OH \times {0.7893 \, g \, C_2H_5OH \over 1 \, ml \, C_2H_5OH} \times {1 \, mole \, C_2H_5OH \over 46.07 \, g\, C_2H_5OH}\] \[ = 0.171 \, mol \, C_2H_5OH \] \[moles \, CH_3CO_2H = {mass \, CH_3CO_2H \over molar \, mass \, CH_3CO_2H} \] \[= {volume \, CH_3CO_2H \times density \, CH_3CO_2H \over molar \, mass \, CH_3CO_2H} \] \[= 10.0 \, ml \, CH_3CO_2H \times {1.0492 \, g \, CH_3CO_2H \over 1 \, ml \, CH_3CO_2H} \times {1 \, mol \, CH_3CO_2H \over 60.05 \, g \, CH_3CO_2H } \] \[= 0.175 \, mol \, CH_3CO_2H \] C The number of moles of acetic acid exceeds the number of moles of ethanol. Because the reactants both have coefficients of 1 in the balanced chemical equation, the mole ratio is 1:1. We have 0.171 mol of ethanol and 0.175 mol of acetic acid, so ethanol is the limiting reactant and acetic acid is in excess. The coefficient in the balanced chemical equation for the product (ethyl acetate) is also 1, so the mole ratio of ethanol and ethyl acetate is also 1:1. This means that given 0.171 mol of ethanol, the amount of ethyl acetate produced must also be 0.171 mol: \[ moles \, ethyl \, acetate = molethanol \times {1 \, mol \, ethyl \, acetate \over 1 \, mol \, ethanol } \] \[ = 0.171 \, mol \, C_2H_5OH \times {1 \, mol \, CH_3CO_2C_2H_5 \over 1 \, mol \, C_2H_5OH} \] \[ = 0.171 \, mol \, CH_3CO_2C_2H_5 \] D The final step is to determine the mass of ethyl acetate that can be formed, which we do by multiplying the number of moles by the molar mass: \[mass \, of \, ethyl \, acetate = moleethyl \, acetate \times molar \, mass \, ethyl \, acetate\] \[ = 0.171 \, mol \, CH_3CO_2C_2H_5 \times {88.11 \, g \, CH_3CO_2C_2H_5 \over 1 \, mol \, CH_3CO_2C_2H_5}\] \[ = 15.1 \, g \, CH_3CO_2C_2H_5 \] Thus 15.1 g of ethyl acetate can be prepared in this reaction. If necessary, you could use the density of ethyl acetate (0.9003 g/cm3) to determine the volume of ethyl acetate that could be produced: \[ volume \, of \, ethyl \, acetate = 15.1 \, g \, CH_3CO_2C_2H_5 \times { 1 \, ml \, CH_3CO_2C_2H_5 \over 0.9003 \, g\, CH_3CO_2C_2H_5} \] \[ = 16.8 \, ml \, CH_3CO_2C_2H_5 \] |
| Exercise 12 |
|---|
| Under appropriate conditions, the reaction of elemental phosphorus and elemental sulfur produces the compound P4S10. How much P4S10 can be prepared starting with 10.0 g of P4 and 30.0 g of S8? Answer: 35.9 g |
Summary
The stoichiometry of a reaction describes the relative amounts of reactants and products in a balanced chemical equation. A stoichiometric quantity of a reactant is the amount necessary to react completely with the other reactant(s). If a quantity of a reactant remains unconsumed after complete reaction has occurred, it is in excess. The reactant that is consumed first and limits the amount of product(s) that can be obtained is the limiting reactant. To identify the limiting reactant, calculate the number of moles of each reactant present and compare this ratio to the mole ratio of the reactants in the balanced chemical equation.
Key Takeaway
The stoichiometry of a balanced chemical equation identifies the maximum amount of product that can be obtained.
| Conceptual Problems |
|---|
|
| Numerical Problems |
|---|
| Please be sure you are familiar with the topics discussed in Essential Skills 2 () before proceeding to the Numerical Problems. 12. Write a balanced chemical equation for each reaction and then determine which reactant is in excess. a. 2.46 g barium(s) plus 3.89 g bromine(l) in water to give barium bromide b. 1.44 g bromine(l) plus 2.42 g potassium iodide(s) in water to give potassium bromide and iodine c. 1.852 g of Zn metal plus 3.62 g of sulfuric acid in water to give zinc sulfate and hydrogen gas d. 0.147 g of iron metal reacts with 0.924 g of silver acetate in water to give iron(II) acetate and silver metal e. 3.142 g of ammonium phosphate reacts with 1.648 g of barium hydroxide in water to give ammonium hydroxide and barium phosphate 13. Under the proper conditions, ammonia and oxygen will react to form dinitrogen monoxide (nitrous oxide, also called laughing gas) and water. Write a balanced chemical equation for this reaction. Determine which reactant is in excess for each combination of reactants. a. 24.6 g of ammonia and 21.4 g of oxygen b. 3.8 mol of ammonia and 84.2 g of oxygen c. 3.6 × 1024 molecules of ammonia and 318 g of oxygen d. 2.1 mol of ammonia and 36.4 g of oxygen 14. When a piece of zinc metal is placed in aqueous hydrochloric acid, zinc chloride is produced, and hydrogen gas is evolved. Write a balanced chemical equation for this reaction. Determine which reactant is in excess for each combination of reactants. a. 12.5 g of HCl and 7.3 g of Zn b. 6.2 mol of HCl and 100 g of Zn c. 2.1 × 1023 molecules of Zn and 26.0 g of HCl d. 3.1 mol of Zn and 97.4 g of HCl 15. Determine the mass of each reactant needed to give the indicated amount of product. Be sure that the chemical equations are balanced. a. NaI(aq) + Cl2(g) → NaCl(aq) + I2(s); 1.0 mol of NaCl b. NaCl(aq) + H2SO4(aq) → HCl(g) + Na2SO4(aq); 0.50 mol of HCl c. NO2(g) + H2O(l) → HNO2(aq) + HNO3(aq); 1.5 mol of HNO3 16. Determine the mass of each reactant needed to give the indicated amount of product. Be sure that the chemical equations are balanced. a. AgNO3(aq) + CaCl2(s) → AgCl(s) + Ca(NO3)2(aq); 1.25 mol of AgCl b. Pb(s) + PbO2(s) + H2SO4(aq) → PbSO4(s) + H2O(l); 3.8 g of PbSO4 c. H3PO4(aq) + MgCO3(s) → Mg3(PO4)2(s) + CO2(g) + H2O(l); 6.41 g of Mg3(PO4)2 17. Determine the percent yield of each reaction. Be sure that the chemical equations are balanced. Assume that any reactants for which amounts are not given are in excess. (The symbol Δ indicates that the reactants are heated.) a. b. Cu(s) + H2SO4(aq) → CuSO4(aq) + SO2(g) + H2O(l); 4.00 g of copper gives 1.2 g of sulfur dioxide c. AgC2H3O2(aq) + Na3PO4(aq) → Ag3PO4(s) + NaC2H3O2(aq); 5.298 g of silver acetate produces 1.583 g of silver phosphate 18. Each step of a four-step reaction has a yield of 95%. What is the percent yield for the overall reaction? 19. A three-step reaction yields of 87% for the first step, 94% for the second, and 55% for the third. What is the percent yield of the overall reaction? 20. Give a general expression relating the theoretical yield (in grams) of product that can be obtained from x grams of B, assuming neither A nor B is limiting. A + 3B → 2C 21. Under certain conditions, the reaction of hydrogen with carbon monoxide can produce methanol. a. Write a balanced chemical equation for this reaction. b. Calculate the percent yield if exactly 200 g of methanol is produced from exactly 300 g of carbon monoxide. 22. Chlorine dioxide is a bleaching agent used in the paper industry. It can be prepared by the following reaction: NaClO2(s) + Cl2(g) → ClO2(aq) + NaCl(aq) a. What mass of chlorine is needed for the complete reaction of 30.5 g of NaClO2? b. Give a general equation for the conversion of x grams of sodium chlorite to chlorine dioxide. 23. The reaction of propane gas (CH3CH2CH3) with chlorine gas (Cl2) produces two monochloride products: CH3CH2CH2Cl and CH3CHClCH3. The first is obtained in a 43% yield and the second in a 57% yield. a. If you use 2.78 g of propane gas, how much chlorine gas would you need for the reaction to go to completion? b. How many grams of each product could theoretically be obtained from the reaction starting with 2.78 g of propane? c. Use the actual percent yield to calculate how many grams of each product would actually be obtained. 24. Protactinium (Pa), a highly toxic metal, is one of the rarest and most expensive elements. The following reaction is one method for preparing protactinium metal under relatively extreme conditions: \[ 2PaI_5 (s) \underrightarrow {\Delta} 2Pa (s) + 5I_2 (s) \] a. Given 15.8 mg of reactant, how many milligrams of protactinium could be synthesized? b. If 3.4 mg of Pa was obtained, what was the percent yield of this reaction? c. If you obtained 3.4 mg of Pa and the percent yield was 78.6%, how many grams of PaI5 were used in the preparation? 25. Aniline (C6H5NH2) can be produced from chlorobenzene (C6H5Cl) via the following reaction: C6H5Cl(l) + 2NH3(g) → C6H5NH2(l) + NH4Cl(s) Assume that 20.0 g of chlorobenzene at 92% purity is mixed with 8.30 g of ammonia. a. Which is the limiting reactant? b. Which reactant is present in excess? c. What is the theoretical yield of ammonium chloride in grams? d. If 4.78 g of NH4Cl was recovered, what was the percent yield? e. Derive a general expression for the theoretical yield of ammonium chloride in terms of grams of chlorobenzene reactant, if ammonia is present in excess. 26. A stoichiometric quantity of chlorine gas is added to an aqueous solution of NaBr to produce an aqueous solution of sodium chloride and liquid bromine. Write the chemical equation for this reaction. Then assume an 89% yield and calculate the mass of chlorine given the following: a. 9.36 × 1024 formula units of NaCl b. 8.5 × 104 mol of Br2 c. 3.7 × 108 g of NaCl |
| Answers |
|---|
| 13. The balanced chemical equation for this reaction is 2NH3 + 2O2 → N2O + 3H2O a. NH3 b. NH3 c. O2 d. NH3 15. a. 150 g NaI and 35 g Cl2 b. 29 g NaCl and 25 g H2SO4 c. 140 g NO2 and 27 g H2O 17. a. 80% b. 30% c. 35.7% 19. 45%. 21. a. CO + 2H2 → CH3OH b. 58.28% 23. a. 2.24 g Cl2 b. 4.95 g c. 2.13 g CH3CH2CH2Cl plus 2.82 g CH3CHClCH3 25. a. chlorobenzene b. ammonia c. 8.74 g ammonium chloride. d. 55% e. \(Theoretical \, yield \, (NH_4Cl) = {mass \, of \, chlorobenzene \, (g) \times 0.92 \times \times 53.49 \, g/mol \over 112.55 \, g/mol} \) |

