3.2 Changes in Concentration
- Page ID
- 32235
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Consider the following equilibrium system:
| \(\ce{Fe^{3+}(aq)}\) | + | \(\ce{SCN^-(aq)}\) | 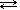 | \(\ce{FeSCN^{2+}(aq)}\) |
| (colourless) | | (red) | ||
If more Fe3+ is added to the reaction, what will happen?
According to Le Châtelier's Principle, the system will react to minimize the stress.
Since Fe3+ is on the reactant side of this reaction, the rate of the forward reaction will increase in order to "use up" the additional reactant. This will cause the equilibrium to shift to the right, producing more FeSCN2+. For this particular reaction we will be able to see that this as happened, as the solution will become a darker red colour.
There are a few different ways we can say what happens here when we add more Fe3+; these all mean the same thing:
- equilibrium shifts to the right
- equilibrium shifts to the product side
- the forward reaction is favoured
How does this cause the concentrations of the reaction participants to change?
| \(\ce{Fe^{3+}}\) | Since this is what was added to cause the stress, the concentration of \(\ce{Fe^{3+}}\) will increase. (a shorthand way to indicate this: \(\ce{[Fe]^{3+}\: \uparrow}\) (Reminder: the square brackets represent "concentration") |
| \(\ce{SCN^{-}(aq)}\) | Equilibrium will shift to the right, which will use up the reactants. The concentration of \(\ce{SCN^{-}(aq)}\) will decrease \(\ce{[SCN]^{-}\: \downarrow}\) as the rate of the forward reaction increases. |
| \(\ce{FeSCN^{2+}}\) | With the forward reaction rate increases, more products are produced, and the concentration of \(\ce{FeSCN^{2+}}\) will increase. \(\ce{[FeSCN]^{2+}} \uparrow \) |
How about the value of Keq? Notice that the concentration of some reaction participants have increased, while others have decreased. Once equilibrium has re-established itself, the value of Keq will be unchanged.
| The value of Keq does not change when changes in concentration cause a shift in equilibrium. |
What if we add more FeSCN2+?
Again, equilibrium will shift to use up the added substance. In this case, equilibrium will shift to favour the reverse reaction, since the reverse reaction will use up the additional FeSCN2+.
- equilibrium shifts to the left
- equilibrium shifts to the reactant side
- the reverse reaction is favoured
How do the concentrations of reaction participants change?
| \(\ce{Fe^{3+}}\) | \(\ce{[Fe]^{3+}\: \uparrow}\) as the reverse reaction is favoured |
| \(\ce{SCN^{-}(aq)}\) | \(\ce{[SCN]^{-}\: \uparrow}\) as the reverse reaction is favoured |
| \(\ce{FeSCN^{2+}}\) | \(\ce{[FeSCN]^{2+}} \uparrow \) because this is the substance that was added |
Concentration can also be changed by removing a substance from the reaction. This is often accomplished by adding another substance that reacts (in a side reaction) with something already in the reaction.
Let's remove SCN- from the system (perhaps by adding some Pb2+ ions - the lead(II) ions will form a precipitate with SCN- , removing them from the solution). What will happen now? Equilibrium will shift to replace SCN- - the reverse reaction will be favoured because that is the direction that produces more SCN-.
- equilibrium shifts to the left
- equilibrium shifts to the reactant side
- the reverse reaction is favoured
How do the concentrations of reaction participants change?
| \(\ce{Fe^{3+}}\) | \(\ce{[Fe]^{3+}\: \uparrow}\) as the reverse reaction is favoured |
| \(\ce{SCN^{-}}\) | \(\ce{[SCN]^{-}\: \uparrow}\) as the reverse reaction is favoured (but it also ↓ because it was removed) |
| \(\ce{FeSCN^{2+}}\) | \(\ce{[FeSCN]^{2+}} \uparrow \) because this is the substance that was added |

