Gas Lasers
- Page ID
- 67373
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)In these lasers the lasing medium is made-up of one or a mixture of gases or vapors. Gas lasers can be classified in terms of the type of transitions that lead to their operation: atomic or molecular. The most common of all gas lasers is the helium-neon (He-Ne) laser. The presence of two atomic species (helium and neon) in this gas laser might suggest that the medium is made of molecules, but these two species of atoms do not form a stable molecule. In fact, all inert atoms like helium, argon, krypton, etc. (those in the last column of the Periodic Table) hold tightly to their own electron clouds and seldom form a molecule or react with other atoms (hence the name: inert). In the He-Ne laser the transition that produces the output light is an atomic transition. Gas lasers that employ molecular gas or vapor for their lasing medium use molecular transitions for their lasing operation. Molecular transitions tend to be more complex than atomic ones. As a consequence the laser light produced by molecular lasers tends to have a wider and more varied collection of properties. Examples of some common molecular gas lasers are carbon monoxide (CO), carbon dioxide (CO2), excimer, and nitrogen (N2) lasers.
Helium Neon (He-Ne) Lasers
The He-Ne laser was the first continuous wave (cw) laser invented. A few months after Maiman announced his invention of the pulsed ruby laser, Ali Javan and his associates W. R. Bennet and D. R. Herriott announced their creation of a cw He-Ne laser. This gas laser is a four-level laser that use helium atoms to excite neon atoms. It is the atomic transitions in the neon that produces the laser light. The most commonly used neon transition in these lasers produces red light at 632.8 nm. But these lasers can also produce green and yellow light in the visible as well as UV and IR (Javan's first He-Ne operated in the IR at 1152.3 nm). By using highly reflective mirrors designed for one of these many possible lasing transitions, a given He-Ne's output is made to operate at a single wavelength.
He-Ne lasers typically produce a few to tens of mW (milli-Watt, or \(10^{-3}\) W) of power. They are not sources of high power laser light. Probably one of the most important features of these lasers is that they are highly stable, both in terms of their wavelength (mode stability) and intensity of their output light (low jitter in power level). For these reasons, He-Ne lasers are often used to stabilize other lasers. They are also used in applications, such as holography, where mode stability is important. Until the mid 1990's, He-Ne lasers were the dominant type of lasers produced for low power applications - from range finding to scanning to optical transmission, to laser pointers, etc. Recently, however, other types of lasers, most notably the semiconductor lasers, seem to have won the competition because of reduced costs.
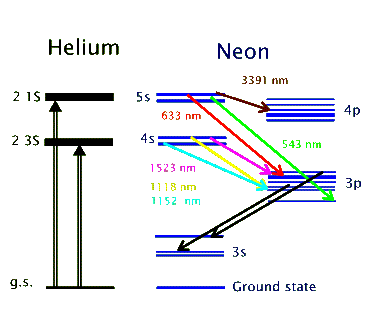
The above energy level diagram shows the two excited states of helium atom, the 2 3S and 2 1S, that get populated as a result of the electromagnetic pumping in the discharge. Both of these states are metastable and do not allow de-excitations via radiative transitions. Instead, the helium atoms give off their energy to neon atoms through collisional excitation. In this way the 4s and 5s levels in neon get populated. These are the two upper lasing levels, each for a separate set of lasing transitions. Radiative decay from the 5s to the 4s levels are forbidden. So, the 4p and 3p levels serve as the lower lasing levels and rapidly decay into the metastable 3s level. In this way population inversion is easily achieved in the He-Ne. The 632.8 nm laser transition, for example, involves the 5s and 3p levels, as shown above.
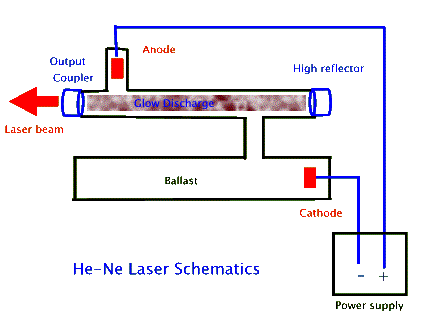
In most He-Ne lasers the gas, a mixture of 5 parts helium to 1 part neon, is contained in a sealed glass tube with a narrow (2 to 3 mm diameter) bore that is connected to a larger size tube called a ballast, as shown above. Typically the laser's optical cavity mirrors, the high reflector and the output coupler, form the two sealing caps for the narrow bore tube. High voltage electrodes create a narrow electric discharge along the length of this tube, which then leads to the narrow beam of laser light. The function of the ballast is to maintain the desired gas mixture. Since some of the atoms may get imbedded in the glass and/or the electrodes as they accelerate within the discharge, in the absence of a ballast the tube would not last very long. To further prolong tube lifetime some of these lasers also use "getters", often metals such as titanium, that absorb impurities in the gas.
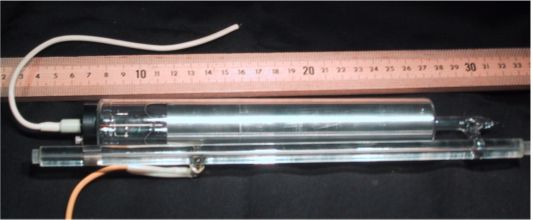
Above photograph shows a commercial He-Ne tube. The thicker cylinder closest to the meter-stick (shown for scale) is the ballast. The thinner tube houses the resonant cavity where the lasing occurs. Notice the two mirrors that seal the two ends of the bore. For mode stability reasons, these mirrors are concave; they serve as the output coupler and the high reflector.
A typical commercially available He-Ne produces about a few mW of 632.8 nm light with a beam width of a few millimeters at an overall efficiency of near 0.1%. This means that for every 1 Watt of input power from the power supply, 1 mW of laser light is produced. Still, because of their long operating lifetime of 20,000 hours or more and their relatively low manufacturing cost, He-Ne lasers are among the most popular gas lasers.
Argon-ion Lasers
Another commonly used gas laser is the argon-ion laser. In these lasers, as in the He-Ne the lasing transition type is atomic. But instead of a neutral atom, here the lasing is the result of the de-excitations of the ion. It takes more energy to ionize an atom than to excite it. By the same token, more energy can be obtained from the de-excitation of the ion. So, doubly (Ar++) and singly ionized (Ar+) argon atoms can radiate shorter wavelength light than could the neutral argon atom, Ar. Because of this, argon-ion lasers can produce uv light with a wavelength as short as 334 nm. In addition, these lasers can produce much more power than He-Ne lasers. Argon-ion lasers typically range in output power from one to as much as 20 W. At the higher power levels their output is multi-mode, i.e. contains several distinct wavelengths. Some of these wavelengths are:
- 334 nm, UV
- 351 nm, UV
- 364 nm, UV
- 458 nm, violet
- 477 nm , violet
- 488 nm, (strong) blue
- 497 nm, blue-green
- 514 nm, (strongest) green
Because of these two reasons, high power and multicolor output, argon-ion is one of the most commonly used lasers in laser light shows, as well as in a variety of applications.
The make-up of a typical argon-ion laser is very similar to a He-Ne's, but with a few slight differences. First, these lasers are much larger in size. A typical Ar++ laser tube is about one meter long, as compared to just 20 cm for that of a He-Ne. Second, the optical cavity of these lasers is built external to the tube. This is partly because of the high power operation of the laser and partly because such external arrangement allows for the use of optional wavelength selection optics within the optical cavity. A prism or a diffraction grating located just before the high reflecting mirror selects only one of the lasing transitions for amplification within the cavity; other wavelengths are deflected out of the resonant cavity. In this way these ion lasers can operate in a so called single mode.

With this arrangement the two mirrors holders on opposite sides of the laser tube are typically attached together with an invar rod for thermal stabilization (Invar is a steel alloy that contains nickel). Its most valued property is that it expands and contracts very little when its temperature changes. As a result, when the laser's temperature changes as it heats up due to the large electric current within the electromagnetic pump discharge, the optical path length, and therefore the modal character of the laser output, remains relatively unchanged. Finally, because of their high power argon-ion lasers require active cooling. This is most commonly accomplished by circulating water, either directly from tap or from commercially produced chillers, in closed coils that surround the plasma (a gas of charged ions) tube and parts of the electric power supply. Some of the lower powered argon-ion lasers are just air cooled using a fan, which makes them less cumbersome to use.
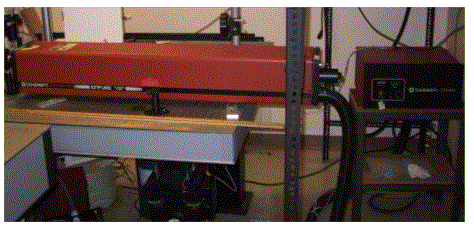
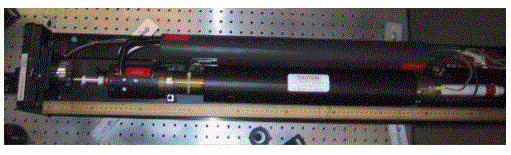
The above two photographs show a 5 W argon-ion laser. Notice the one-meter long laser tube, the large ballast, and the umbilical cord that connects to the laser power supply. This cord contains not only the power line that supplies the laser with the electric power to generate the plasma, but also the water lines that circulate water to cool the laser.
Another type of ion laser, the krypton laser, operates very much the same as the argon-ion laser. To take advantage of all the colors available in both argon and krypton lasers, manufacturers make argon-krypton ion lasers by using a suitable mixture of these two gases. The mixed gas lasers are very useful for entertainment applications because, in addition to many colors, they can also produce a "white" beam. (Why is the word "white" in quotations?)
Carbon-dioxide (\(CO_2\)) and Carbon-monoxide (\(CO\)) Lasers
In both of these lasers the gaseous medium is made-up of molecules, which in addition to electronic energy levels of atoms also have both molecular vibrational and rotational energy levels. The vibrational energy levels are similar to finer spaced ladder rungs that span two rungs of the electronic energy levels. The rotational levels are still more finely spaced rungs that span the vibrational rungs! In these gas lasers the lasing transitions occur among the vibrational levels, typically belonging to different electronic levels.
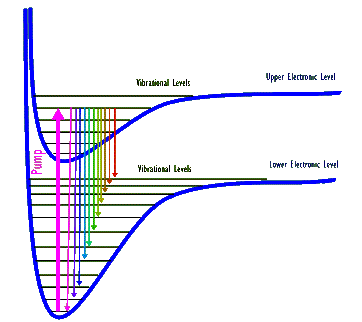
Above diagram shows two electronic and several of their associated vibrational levels for a hypothetical molecule. (Electronic levels are shown as "bent rungs" because in the molecule atoms can change their separation distance and therefore their electronic energy. Also, note that rotational levels are not shown.) A thick arrow depicts a pump that excites the molecule from its lowest vibrational level belonging to the lower electronic level to the 5th highest vibrational level of the next upper electronic level. The excited molecule can then de-excite out of this upper level into many possible vibrational levels. Each one of these de-excitations produces a photon whose energy, and therefore its wavelength, corresponds to that specific de-excitation. As a result, when a collection of these molecules are all excited by this pump they generate a number (eleven, in this drawing) of different wavelength photons.
Specifically, \(CO_2\) lasers can generate an output wavelength from about 9 micro-meters (mm, or microns) to about 11 microns (1 micron is one millionth of a meter, or 1000 nm.) These outputs generally contain many closely spaced wavelengths, if the laser is used for high power output. But for more wavelength specific applications the optical cavity of the laser is designed to amplify just one or a few of the vibrational radiative decay lines. The wavelength range for the CO laser is lower, from about 5 to 6 mm. Another feature of these gas lasers that make them one of the most versatile of all gas lasers is that they can be made to operate over a large range of power outputs, either in a pulsed or cw mode. The \(CO_2\) laser, in particular, ranges in cw power from few Watts to kWs, making these lasers ideal for many industrial applications including welding and drilling.
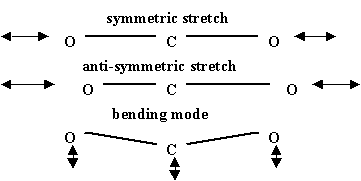
Molecular vibrations for \(CO_2\), a linear molecule, are shown in the figure below. Other combinations of these are possible but these three are fundamental. There are different varieties of \(CO_2\) lasers that flow fresh gases through the resonant cavity area in order both to remove heat and to provide lots of gas to achieve high laser powers. For these lasers in the cw mode powers can reach as high as 100 kW. These intense laser beams are essentially tremendous invisible "heat" beams that can cut through thick pieces of metal and are used extensively in industrial applications.
We mention two interesting tidbits about these lasers. First, since glass in not very transparent to IR light, the mirrors are actually made of special crystalline materials that are transparent to the IR. Second, recall that IR light is invisible to our eyes and so special precautions are needed to protect people working around these lasers. It turns out that although these lasers can easily cut through metal, they cannot pass through a thin sheet of clear plexiglass, and so often these systems are housed in a plexiglass shell to block any stray reflected IR light.
Other types of gas lasers include the nitrogen laser (N2), excimers, copper-vapor, gold-vapor, and chemical lasers. Of these the excimer lasers and the chemical lasers are the most different from the ones we have already discussed above.
Excimer Lasers
Similar to \(CO_2\), \(CO\), and \(N_2\) lasers, these gas lasers also use molecular transitions for their lasing operation. What makes them especially different is that the molecular gas used for these lasers has no ground state! Typically these molecules include an atom belonging to the inert gas family (argon, xenon, krypton) and one from the halide group (chlorine, fluorine, and bromine). The inert gas atoms (also known as the rare gases) do not want to interact with any other atoms. On the other hand, the halide gases are highly reactive. Still, they cannot bond with the inert gases to form a molecule. But when sufficient energy is provided to these atoms they bind together in a short-lived excited state that soon (few nanoseconds) decays back into the original two separate atoms (i.e. the molecule dissociates). Because of this rapid molecular dissociation, these lasers obtain population inversion just by excitation alone! In fact, the word excimer is short for "excited dimer," although most excimer lasers do not use two identical atoms as a strict dimer would.
The excimer molecules are created from a mixture of inert gases along with one of the halides. Typically a few percent of Ar, Kr and Xe are mixed with a few percent of a halide to form excimer molecules: ArF, KrF , and XeF. The other 90% of the gas mix consists of other inert gases such as He and Ne which act simply as a buffer and do not take part in the reaction. A large electric pulse is often used for the excitation and formation of the excimer molecule. The rapid decay of the short-lived molecule then leads to a very short laser pulse lasting 10 - 100 ns (10-8 - 10-7 s). So, another unusual feature of the excimers is that they do not require an optical amplifier. They are very efficiently formed in the reaction with an efficiency of around 30% so that the gain is extremely high. A high reflector and a glass (really quartz - why?) window is sufficient for laser light production. This means that only about 4% of the light is reflected back into the cavity at the front window, but the gain is so high that only a single pass through the cavity is needed to produce lots of uv light. Typically about 1 Joule of energy is in a 10 ns pulse, so that the pulse power is 1J/10ns = 100 MW. If this power were steadily produced it would be equivalent to powers generated by large power plants. However, only about 1 - 100 pulses are produced per second, so that the average power produced is about 1 - 100 W.
Because of the highly reactive nature of the halide gas used in these lasers, excimers are not very easy to operate. The halides tend to be very corrosive and therefore add a great deal to the operational cost (as well as the danger!) of these lasers. But still these lasers are very much in use because their output wavelength is in the UV, from 350 nm down to as low as 193 nm; all with a good deal of power.
Chemical Lasers
In these lasers the energy of excitation comes from a chemical reaction that takes place in the medium itself. In this sense, then, chemical lasers are self-pumped. Even more interesting is the operation of one of the most common of these lasers, the hydrogen-fluoride laser that operates in the IR at 4.6 microns. The added intrigue is due to the chain reaction that takes place to excite the laser molecule, \(\ce{HF}\). A mixture of hydrogen (molecular) gas, \(\ce{H2}\), and fluorine gas, \(\ce{F_2}\), is subjected to an electric discharge to start the chain reaction, which results in the production of a hydrogen-fluoride molecule in an excited vibrational level (excited state is denoted by a starred superscript: \(HF^*\)) and the dissociative production of H or F for the next reaction:
\[\ce{F + H_2 \rightarrow HF^{*} + H} \nonumber \]
\[\ce{F2 + H \rightarrow HF^{*} + F} \nonumber \]
\[\ce{F + H2 \rightarrow HF^{*} + H} \nonumber \]
\[\ce{F_2 + H \rightarrow HF^{*} + F} \nonumber \]
etc.
So, the medium in these lasers is used up as a fuel to generate their laser light. Therefore, practical limitations aside, the power of these lasers depends on the amount of the chemical (gas volume) that is used in the laser. Because of their conceivably limitless power output, these lasers have been studied mostly for their military applications. The most famous of these is the US Army's Mid-Infrared Advanced Chemical Laser, MIRACL, located at White Sands Missile Range, New Mexico. This is a formidable chemical laser that has been developed as part of the Department of Defense's efforts for its Strategic Defense Initiative (SDI).
MIRACL is the US's most powerful laser. It operates in a band from 3.6 to 4.2 microns producing megawatts of CW output for as long as 70 seconds. For its fuel it burns ethylene, \(C_2H_4\), with nitrogen trifluoride, NF3. The resulting free fluorine atoms combine with deuterium gas that is injected to this burning fuel to form deuterium fluoride molecules (DF), which ultimately provide the laser light. In this laser the beam within the optical cavity is about 21 cm high and 3 cm wide, resulting in a 14 cm2 output beam. For testing purposes this laser is used with an aiming and focusing telescope system called the SEALITE Beam Director (SLBD) which was first developed by the Navy. The moving part of SLBD that is capable of fast rotations and high accelerations weighs about 18,000 pounds. Its telescope can focus the beam to any target that is located within a range of a minimum of 400 meters up to infinity.
Contributors
Jay Newman and Seyffie Maleki (Union College)

