Electron Configuration
- Page ID
- 1701
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)The electron configuration of an atomic species (neutral or ionic) allows us to understand the shape and energy of its electrons. Many general rules are taken into consideration when assigning the "location" of the electron to its prospective energy state, however these assignments are arbitrary and it is always uncertain as to which electron is being described. Knowing the electron configuration of a species gives us a better understanding of its bonding ability, magnetism and other chemical properties.
Introduction
The electron configuration is the standard notation used to describe the electronic structure of an atom. Under the orbital approximation, we let each electron occupy an orbital, which can be solved by a single wavefunction. In doing so, we obtain three quantum numbers (n,l,ml), which are the same as the ones obtained from solving the Schrödinger's equation for Bohr's hydrogen atom. Hence, many of the rules that we use to describe the electron's address in the hydrogen atom can also be used in systems involving multiple electrons. When assigning electrons to orbitals, we must follow a set of three rules: the Aufbau Principle, the Pauli-Exclusion Principle, and Hund's Rule.
The wavefunction is the solution to the Schrödinger equation. By solving the Schrödinger equation for the hydrogen atom, we obtain three quantum numbers, namely the principal quantum number (n), the orbital angular momentum quantum number (l), and the magnetic quantum number (ml). There is a fourth quantum number, called the spin magnetic quantum number (ms), which is not obtained from solving the Schrödinger equation. Together, these four quantum numbers can be used to describe the location of an electron in Bohr's hydrogen atom. These numbers can be thought of as an electron's "address" in the atom.
Notation
To help describe the appropriate notation for electron configuration, it is best to do so through example. For this example, we will use the iodine atom. There are two ways in which electron configuration can be written:
I: 1s22s22p63s23p64s23d104p65s24d105p5
or
I: [Kr]5s24d105p5
In both of these types of notations, the order of the energy levels must be written by increased energy, showing the number of electrons in each subshell as an exponent. In the short notation, you place brackets around the preceding noble gas element followed by the valence shell electron configuration. The periodic table shows that kyrpton (Kr) is the previous noble gas listed before iodine. The noble gas configuration encompases the energy states lower than the valence shell electrons. Therefore, in this case [Kr]=1s22s22p63s23p64s23d104p6.
Quantum Numbers
Principal Quantum Number (n)
The principal quantum number n indicates the shell or energy level in which the electron is found. The value of n can be set between 1 to n, where n is the value of the outermost shell containing an electron. This quantum number can only be positive, non-zero, and integer values. That is, n=1,2,3,4,..
For example, an Iodine atom has its outmost electrons in the 5p orbital. Therefore, the principle quantum number for Iodine is 5.
Orbital Angular Momentum Quantum Number (l)
The orbital angular momentum quantum number, l, indicates the subshell of the electron. You can also tell the shape of the atomic orbital with this quantum number. An s subshell corresponds to l=0, a p subshell = 1, a d subshell = 2, a f subshell = 3, and so forth. This quantum number can only be positive and integer values, although it can take on a zero value. In general, for every value of n, there are n values of l. Furthermore, the value of l ranges from 0 to n-1. For example, if n=3, l=0,1,2.
So in regards to the example used above, the l values of Iodine for n = 5 are l = 0, 1, 2, 3, 4.
Magnetic Quantum Number (ml)
The magnetic quantum number, ml, represents the orbitals of a given subshell. For a given l, ml can range from -l to +l. A p subshell (l=1), for instance, can have three orbitals corresponding to ml = -1, 0, +1. In other words, it defines the px, py and pzorbitals of the p subshell. (However, the ml numbers don't necessarily correspond to a given orbital. The fact that there are three orbitals simply is indicative of the three orbitals of a p subshell.) In general, for a given l, there are 2l+1 possible values for ml; and in a n principal shell, there are n2 orbitals found in that energy level.
Continuing on from out example from above, the ml values of Iodine are ml = -4, -3, -2, -1, 0 1, 2, 3, 4. These arbitrarily correspond to the 5s, 5px, 5py, 5pz, 4dx2-y2, 4dz2, 4dxy, 4dxz, and 4dyz orbitals.
Spin Magnetic Quantum Number (ms)
The spin magnetic quantum number can only have a value of either +1/2 or -1/2. The value of 1/2 is the spin quantum number, s, which describes the electron's spin. Due to the spinning of the electron, it generates a magnetic field. In general, an electron with a ms=+1/2 is called an alpha electron, and one with a ms=-1/2 is called a beta electron. No two paired electrons can have the same spin value.
Out of these four quantum numbers, however, Bohr postulated that only the principal quantum number, n, determines the energy of the electron. Therefore, the 3s orbital (l=0) has the same energy as the 3p (l=1) and 3d (l=2) orbitals, regardless of a difference in l values. This postulate, however, holds true only for Bohr's hydrogen atom or other hydrogen-like atoms.
When dealing with multi-electron systems, we must consider the electron-electron interactions. Hence, the previously described postulate breaks down in that the energy of the electron is now determined by both the principal quantum number, n, and the orbital angular momentum quantum number, l. Although the Schrödinger equation for many-electron atoms is extremely difficult to solve mathematically, we can still describe their electronic structures via electron configurations.
General Rules of Electron Configuration
There are a set of general rules that are used to figure out the electron configuration of an atomic species: Aufbau Principle, Hund's Rule and the Pauli-Exclusion Principle. Before continuing, it's important to understand that each orbital can be occupied by two electrons of opposite spin (which will be further discussed later). The following table shows the possible number of electrons that can occupy each orbital in a given subshell.
| subshell | number of orbitals | total number of possible electrons in each orbital |
| s | 1 | 2 |
| p | 3 (px, py, pz) | 6 |
| d | 5 (dx2-y2, dz2, dxy, dxz, dyz) | 10 |
| f | 7 (fz3, fxz2, fxyz, fx(x2-3y2), fyz2, fz(x2-y2), fy(3x2-y2) |
14 |
Using our example, iodine, again, we see on the periodic table that its atomic number is 53 (meaning it contains 53 electrons in its neutral state). Its complete electron configuration is 1s22s22p63s23p64s23d104p65s24d105p5. If you count up all of these electrons, you will see that it adds up to 53 electrons. Notice that each subshell can only contain the max amount of electrons as indicated in the table above.
Aufbau Principle
The word 'Aufbau' is German for 'building up'. The Aufbau Principle, also called the building-up principle, states that electron's occupy orbitals in order of increasing energy. The order of occupation is as follows:
1s<2s<2p<3s<3p<4s<3d<4p<5s<4d<5p<6s<4f<5d<6p<7s<5f<6d<7p
Another way to view this order of increasing energy is by using Madelung's Rule:
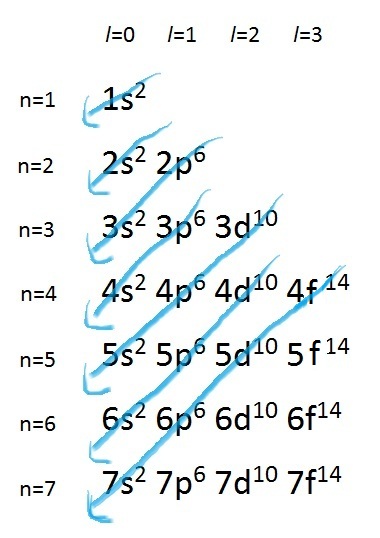
Figure 1. Madelung's Rule is a simple generalization which
dictates in what order electrons should be filled in the
orbitals, however there are exceptions such as
copper and chromium.
This order of occupation roughly represents the increasing energy level of the orbitals. Hence, electrons occupy the orbitals in such a way that the energy is kept at a minimum. That is, the 7s, 5f, 6d, 7p subshells will not be filled with electrons unless the lower energy orbitals, 1s to 6p, are already fully occupied. Also, it is important to note that although the energy of the 3d orbital has been mathematically shown to be lower than that of the 4s orbital, electrons occupy the 4s orbital first before the 3d orbital. This observation can be ascribed to the fact that 3d electrons are more likely to be found closer to the nucleus; hence, they repel each other more strongly. Nonetheless, remembering the order of orbital energies, and hence assigning electrons to orbitals, can become rather easy when related to the periodic table.
To understand this principle, let's consider the bromine atom. Bromine (Z=35), which has 35 electrons, can be found in Period 4, Group VII of the periodic table. Since bromine has 7 valence electrons, the 4s orbital will be completely filled with 2 electrons, and the remaining five electrons will occupy the 4p orbital. Hence the full or expanded electronic configuration for bromine in accord with the Aufbau Principle is 1s22s22p63s23p64s23d104p5. If we add the exponents, we get a total of 35 electrons, confirming that our notation is correct.
Hund's Rule
Hund's Rule states that when electrons occupy degenerate orbitals (i.e. same n and l quantum numbers), they must first occupy the empty orbitals before double occupying them. Furthermore, the most stable configuration results when the spins are parallel (i.e. all alpha electrons or all beta electrons). Nitrogen, for example, has 3 electrons occupying the 2p orbital. According to Hund's Rule, they must first occupy each of the three degenerate p orbitals, namely the 2px orbital, 2py orbital, and the 2pz orbital, and with parallel spins (Figure 2). The configuration below is incorrect because the third electron occupies does not occupy the empty 2pz orbital. Instead, it occupies the half-filled 2px orbital. This, therefore, is a violation of Hund's Rule (Figure 2).
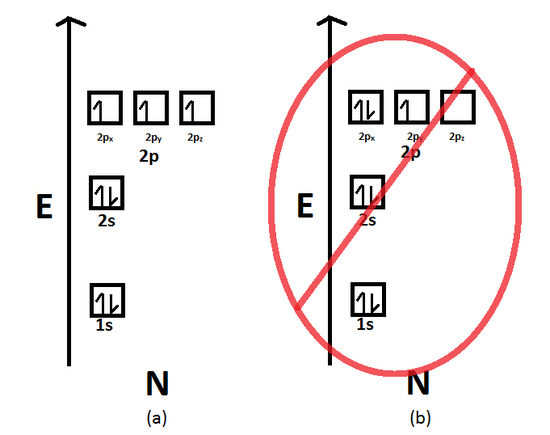
Figure 2. A visual representation of the Aufbau Principle and Hund's Rule. Note that the filling of electrons in each orbital
(px, py and pz) is arbitrary as long as the electrons are singly filled before having two electrons occupy the same orbital.
(a)This diagram represents the correct filling of electrons for the nitrogen atom. (b) This diagramrepresents the incorrect
filling of the electrons for the nitrogen atom.
Pauli-Exclusion Principle
Wolfgang Pauli postulated that each electron can be described with a unique set of four quantum numbers. Therefore, if two electrons occupy the same orbital, such as the 3s orbital, their spins must be paired. Although they have the same principal quantum number (n=3), the same orbital angular momentum quantum number (l=0), and the same magnetic quantum number (ml=0), they have different spin magnetic quantum numbers (ms=+1/2 and ms=-1/2).
Electronic Configurations of Cations and Anions
The way we designate electronic configurations for cations and anions is essentially similar to that for neutral atoms in their ground state. That is, we follow the three important rules: Aufbau Principle, Pauli-exclusion Principle, and Hund's Rule. The electronic configuration of cations is assigned by removing electrons first in the outermost p orbital, followed by the s orbital and finally the d orbitals (if any more electrons need to be removed). For instance, the ground state electronic configuration of calcium (Z=20) is 1s22s22p63s23p64s2. The calcium ion (Ca2+), however, has two electrons less. Hence, the electron configuration for Ca2+ is 1s22s22p63s23p6. Since we need to take away two electrons, we first remove electrons from the outermost shell (n=4). In this case, all the 4p subshells are empty; hence, we start by removing from the s orbital, which is the 4s orbital. The electron configuration for Ca2+ is the same as that for Argon, which has 18 electrons. Hence, we can say that both are isoelectronic.
The electronic configuration of anions is assigned by adding electrons according to Aufbau Principle. We add electrons to fill the outermost orbital that is occupied, and then add more electrons to the next higher orbital. The neutral atom chlorine (Z=17), for instance has 17 electrons. Therefore, its ground state electronic configuration can be written as 1s22s22p63s23p5. The chloride ion (Cl-), on the other hand, has an additional electron for a total of 18 electrons. Following Aufbau Principle, the electron occupies the partially filled 3p subshell first, making the 3p orbital completely filled. The electronic configuration for Cl- can, therefore, be designated as 1s22s22p63s23p6. Again, the electron configuration for the chloride ion is the same as that for Ca2+ and Argon. Hence, they are all isoelectronic to each other.
Problems
1. Which of the princples explained above tells us that electrons that are paired cannot have the same spin value?
2. Find the values of n, l, ml, and ms for the following:
a. Mg
b. Ga
c. Co
3. What is a possible combination for the quantum numbers of the 5d orbital? Give an example of an element which has the 5d orbital as it's most outer orbital.
4. Which of the following cannot exist (there may be more than one answer):
a. n = 4; l = 4; ml = -2; ms = +1/2
b. n = 3; l = 2; ml = 1; ms = 1
c. n = 4; l = 3; ml = 0; ms = +1/2
d. n = 1; l = 0; ml = 0; ms = +1/2
e. n = 0; l = 0; ml = 0; ms = +1/2
5. Write electron configurations for the following:
a. P
b. S2-
c. Zn3+
Answers
1. Pauli-exclusion Principle
2. a. n = 3; l = 0, 1, 2; ml = -2, -1, 0, 1, 2; ms can be either +1/2 or -1/2
b. n = 4; l = 0, 1, 2, 3; ml = -3, -2, -1, 0, 1, 2, 3; ms can be either +1/2 or -1/2
c. n = 3; l = 0, 1, 2; ml = -2, -1, 0, 1, 2, 3; ms can be either +1/2 or -1/2
3. n = 5; l = 3; ml = 0; ms = +1/2. Osmium (Os) is an example.
4. a. The value of l cannot be 4, because l ranges from (0 - n-1)
b. ms can only be +1/2 or -1/2
c. Okay
d. Okay
e. The value of n cannot be zero.
5. a. 1s22s22p63s23p3
b. 1s22s22p63s23p6
c. 1s22s22p63s23p64s23d7
References
- Atkins, P. W., & De Paula, J. (2006). Physical Chemistry for the Life Sciences. New York, NY: W. H. Freeman and Company.
- Petrucci, R. H., Harwood, W. S., & Herring, F. G. (2002). General Chemistry: Principles and Modern Applications. Upper Saddle River, NJ: Prentice-Hall, Inc.
- Shagoury, Richard. Chemistry 1A Lecture Book. 4th Ed. Custom Publishing. 2006. Print
Contributors and Attributions
- Lannah Lua, Andrew Iskandar (University of California Davis, Undergraduate) Mary Magsombol (University of California Davis)

