4.12: Steady-State Approximation
- Page ID
- 35450
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Explain steady state and steady-state approximation.
- Derive a rate law when a mechanism is given but the rate determining step is not identified.
- Derive a general expression of the rate law using the steady-state approximation.
- Make appropriate assumptions so that the derived rate law agrees with the observed rate law.
- Give expressions for the producing rate of an intermediate.
- Give expressions for the consuming rate of an intermediate.
- Express concentration of intermediate in terms of concentration of reactants.
- Eliminate concentrations of intermediates using concentrations of reactants.
- Derive a rate law from the many elementary steps.
- Discuss the derived rate law.
The Steady-State Approximation
When a reaction mechanism has several steps of comparable rates, the rate-determining step is often not obvious. However, there is an intermediate in some of the steps. An intermediate is a species that is neither one of the reactants, nor one of the products. The steady-state approximation is a method used to derive a rate law. The method is based on the assumption that one intermediate in the reaction mechanism is consumed as quickly as it is generated. Its concentration remains the same in a duration of the reaction.
An intermediate is a species that is neither one of the reactants, nor one of the products. It transiently exists during the course of the reaction.
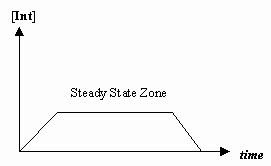
When a reaction involves one or more intermediates, the concentration of one of the intermediates remains constant at some stage of the reaction. Thus, the system has reached a steady-state. The concentration of one of the intermediates, \([Int]\), varies with time as shown in Figure \(\PageIndex{1}\). At the start and end of the reaction, [Int] does vary with time.
\[ \dfrac{d[Int]}{dt}= 0 \nonumber \]
When a reaction mechanism has several steps with comparable rates, the rate-determining step is not obvious. However, there is an intermediate in some of the steps. The steady-state approximation implies that you select an intermediate in the reaction mechanism, and calculate its concentration by assuming that it is consumed as quickly as it is generated. In the following, an example is given to show how the steady-state approximation method works.
Use the steady-state approximation to derive the rate law for this reaction
\[\ce{2 N2O5 \rightarrow 4 NO2 + O2}\nonumber \]
assuming it follows the following three-step mechanism:
\[\begin{align} \ce{N_2O_5} &\underset{\Large{k_{\textrm b}}}{\overset{\Large{k_{\textrm f}}}\rightleftharpoons} \ce{NO_2 + NO_3} \tag{step 1} \\[4pt] \ce{NO3 + NO2} &\ce{->[\large{k_2}] NO + NO2 + O2} \tag{step 2} \\[4pt] \ce{NO3 + NO} & \ce{->[\Large{k_3}] 2 NO2} \tag{step 3} \end{align} \]
Solution
In these steps, \(\ce{NO}\) and \(\ce{NO3}\) are intermediates. You have
\(\ce{production\: rate\: of\: NO} = k_2 \ce{[NO3] [NO2]}\)
\(\ce{consumption\: rate\: of\: NO} = k_3 \ce{[NO3] [NO]}\)
A steady-state approach makes use of the assumption that the rate of production of an intermediate is equal to the rate of its consumption. Thus, we have
\(k_2 \ce{[NO3] [NO2]} = k_3 \ce{[NO3] [NO]}\)
and solving for \(\ce{[NO]}\) gives the result,
\(\ce{[NO]} = \dfrac{k_2 \ce{[NO3] [NO2]}}{k_3 \ce{[NO3]}} \tag{1}\)
For the other intermediate \(\ce{NO3}\),
\(\ce{production\: rate\: of\: NO3} = k_{\ce f} \ce{[N2O5]}\)
\(\ce{consumption\: rate\: of\: NO3} = k_2\ce{[NO3] [NO2]} + k_3\ce{[NO3] [NO]} + k_{\ce b}\ce{[NO3] [NO2]}\)
Applying the steady-state assumption gives:
\(k_{\ce f} \ce{[N2O5]} = k_2\ce{[NO3] [NO2]} + k_3\ce{[NO3] [NO]} + k_{\ce b}\ce{[NO3] [NO2]}\)
Thus,
\(\ce{[NO3]} = \dfrac{k_{\ce f} \ce{[N2O5]}}{k_2\ce{[NO2]} + k_3\ce{[NO]} + k_{\ce b}\ce{[NO2]}}\tag{2}\)
Let's review the three equations (steps) in the mechanism:
Step i. is at equilibrium and thus can not give a rate expression.
Step ii. leads to the production of some products, and the active species \(\ce{NO}\) causes further reaction in step iii. This consideration led to a rate expression from step ii. as:
\(\ce{\dfrac{d[O2]}{dt}} = k_2 \ce{[NO3] [NO2]} \tag{3}\)
Substituting (1) in (2) and then in (3) gives
\(\ce{\dfrac{d[O2]}{dt}}= \dfrac{k_{\ce f} k_2 \ce{[N2O5]}}{k_{\ce b} + 2 k_2} = \ce{k [N2O5]}\)
where \(\ce{k} = \dfrac{k_{\ce f} k_2}{k_{\ce b} + 2 k_2}\).
This is the differential rate law, and it agrees with the experimental results. Carry out the above manipulation yourself on a piece of paper. Simply reading the above will not lead to solid learning yet.
This page gives another example to illustrate the technique of deriving rate laws using the steady-state approximation. The reaction considered here is between \(\ce{H2}\) and \(\ce{I2}\) gases.
For the reaction:
\(\ce{H_{2\large{(g)}} + I_{2\large{(g)}} \rightarrow 2 HI_{\large{(g)}}}\)
what mechanisms might be appropriate? Derive a rate law from the proposed mechanism.
Solution
Well, this question does not have a simple answer, and there is no way to prove one over another for its validity. Beginning chemistry students will not be asked to propose a mechanism, but you will be asked to derive the rate law from the proposed mechanism.
First of all, you should be able to express the rate of reaction in terms of the concentration changes,
\(rate = - \ce{\dfrac{d[H2]}{dt}} = - \ce{\dfrac{ d[I2]}{dt}} = \ce{\dfrac{1}{2}\dfrac{d[HI]}{dt}}\)
Look at the overall reaction equation again to see its relationship and the rate expressions.
Proposing a mechanism
In order to propose a mechanism, we apply the following reasoning. Since the bonding between \(\ce{I-I}\) is weak, we expect \(\ce{I2}\) to dissociate into atoms or radicals. These radicals are active, and they react with \(\ce{H2}\) to produce the products. Thus we propose the three-step mechanism:
- \(\ce{I_{2\large{(g)}}} \xrightarrow{\Large{k_1}} \ce{2 I_{\large{(g)}}}\)
- \(\ce{2 I_{\large{(g)}}} \xrightarrow{\Large{k_2}} \ce{I_{2\large{(g)}}}\)
- \(\ce{H_{2\large{(g)}} + 2 I_{\large{(g)}}} \xrightarrow{\Large{k_3}} \ce{2 HI_{\large{(g)}}}\)
Which step would you use to write the differential rate law?
Since only step iii. gives the real products, we expect you to recognize that step iii. hints the rate law to be:
\(rate = k_3 \ce{[H2] [I]^2}\)
However, this is not a proper rate law, because \(\ce{I}\) is an intermediate, not a reactant. So, you have to express \(\ce{[I]}\) or \(\ce{[I]^2}\) in terms of the concentration of reactants. To do this, we use the steady-state approximation and write out the following relationships:
\(\textrm{rate of producing I} = 2 k_1 \ce{[I2]}\)
\(\textrm{rate of consuming I} = 2 k_2 \ce{[I]^2} + 2 k_3 \ce{[H2] [I]^2}\)
\(\textrm{producing rate of I} = \textrm{consuming rate of I}\)
Thus,
\(\ce{[I]^2} = \dfrac{k_1 \ce{[I2]}}{k_2 + k_3 \ce{[H2]}}\)
Substituting this for \(\ce{[I]^2}\) into the rate expression, you have
\(\begin{align}
rate &= k_3 \ce{[H2]} \dfrac{k_1 \ce{[I2]}}{k_2 + k_3 \ce{[H2]}}\\
&= \dfrac{k_1 k_3 \ce{[H2] [I2]}}{k_2 + k_3 \ce{[H2]}}
\end{align}\)
Discussion
If step iii. is slow, then \(k_3\) and \(k_2 >> k_3 \ce{[H2]}\). The rate law is reduced to\(\ce{rate} = \ce{k [H2] [I2]}\),
where \(\ce{k} = \dfrac{k_1 k_3}{k_2}\). (Work this out on paper yourself; reading the above derivation does not lead to learning.)
Since the rate law is first order with respect to both reactants, one may argue that the rate law also supports a one-step mechanism,
\(\ce{H_{2\large{(g)}} + I_{2\large{(g)}} \rightarrow 2 HI}\)
This elementary step is the same as the overall reaction.
Suppose we use a large quantity of \(\ce{H2}\) compared to \(\ce{I2}\), then the change in \(\ce{[H2]}\) is insignificant. For example, if \(\mathrm{[H_2] = 10}\), and \(\mathrm{[I_2] = 0.1}\) initially, \(\ce{[H2]}\) remains essentially 10 (9.9 with only one significant figure). In other words, \(\ce{[H2]}\) hardly changed when the reaction ended. Thus,
\(k_3 \ce{[H2]} >> k_3\)
and the rate law becomes:
\(rate = k_1 \ce{[I2]}\).
Thus, the reaction is a pseudo first order reaction, due to the large quantity of one reactant. The results suggest iii. a fast step (due to large quantity of \(\ce{H2}\)), and i. the rate determining step.
Contributors and Attributions
Chung (Peter) Chieh (Professor Emeritus, Chemistry @ University of Waterloo)

