Chapter 10 Solutions
- Page ID
- 1108
In-chapter exercises
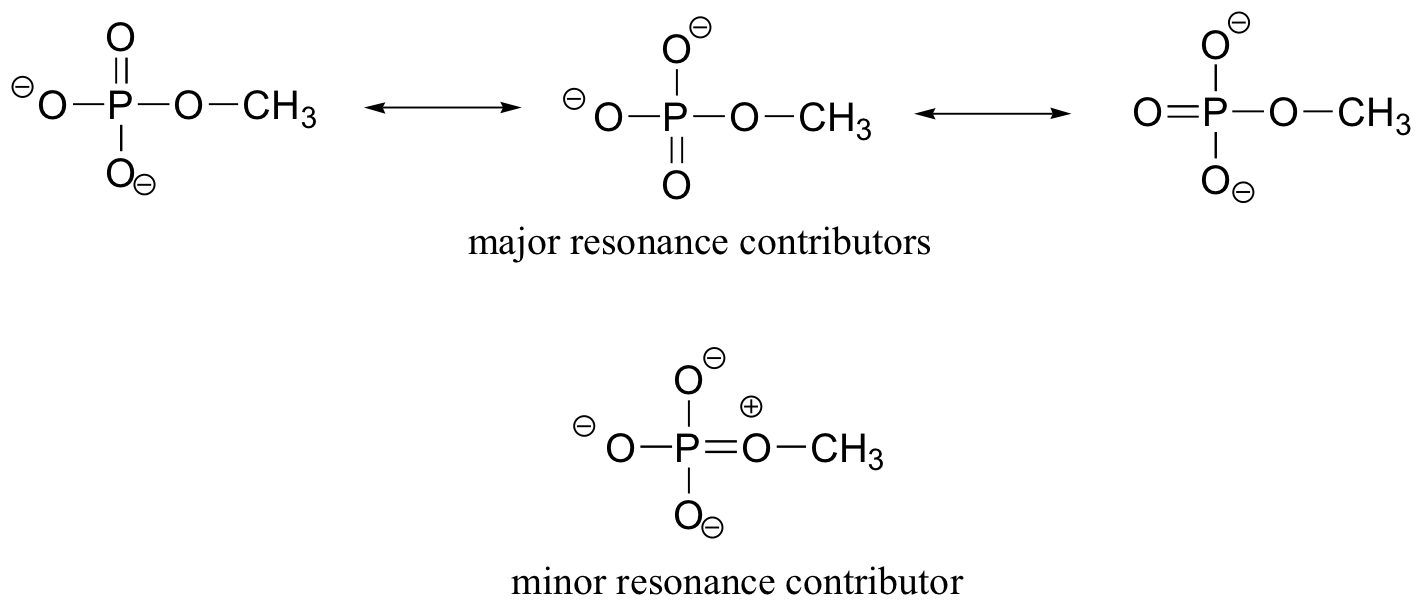
E10.1: We’ll use methyl phosphate as a simple example of a phosphate monoester. The three major resonance forms show how the three non-bridging oxygens share a double bond. Another way of putting this is that each of these bonds is a single s bond plus 1/3 of a pi bond – a bonding order of 1.33. However, if we also consider the minor resonance form in which the bridging oxygen is double-bonded (it's a minor form because of the separation of charge), we can also think of the double bond being shared, in a small part, by the bridging oxygen. We can approximate these ideas by saying that the bridging bond order is slightly more than 1, and the non-bridging bond order is slightly less than 1.33.
E10.2:
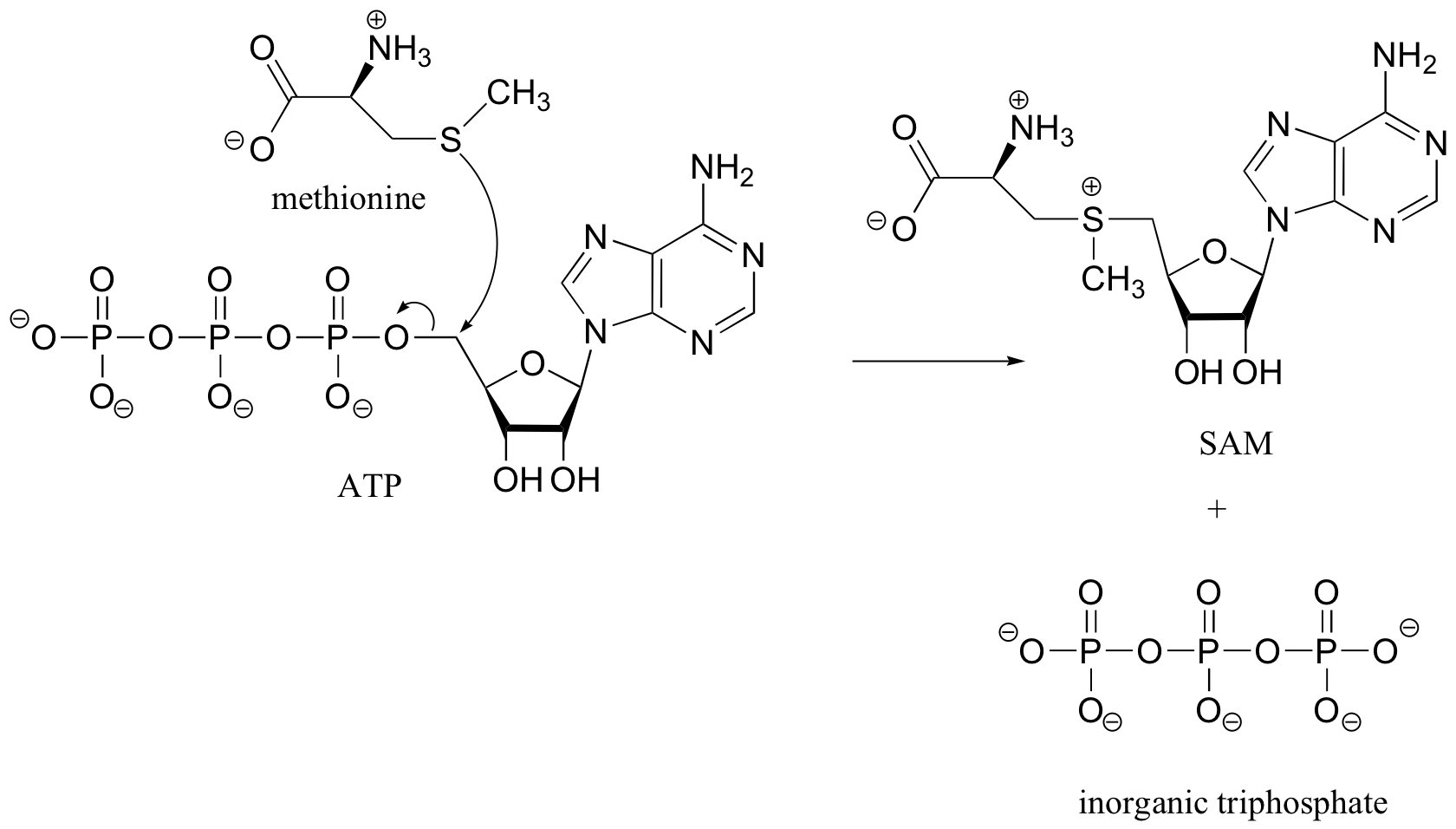
A,E, and I are all representations of an alkyl- or acyl-adenosine phosphate ester (the aspartyl adenosine phosphate species in the first figure of section 10.2E is an actual example of an acyl-adenosine phosphate ester)
F and N are both representations of an organic monophosphate ester, such as glucose-6-phosphate (section 10.2B).
C and G are both representations of an organic diphosphate ester, such as mevalonate 5-diphosphate (section 10.2C).
B, H, K, R, and S are all representations of adenosine triphosphate (ATP – section 10.2A).
D, L and O are all representations of inorganic diphosphate (also known as inorganic pyrophosphate).
J, M, P, and Q are all representations of adenosine diphosphate (ADP).
E10.3: We can use a number of alternative abbreviations for ATP and ADP in showing this mechanism, but the ones used below are relatively compact and still show the chemistry taking place at the gamma-phosphate of ATP:
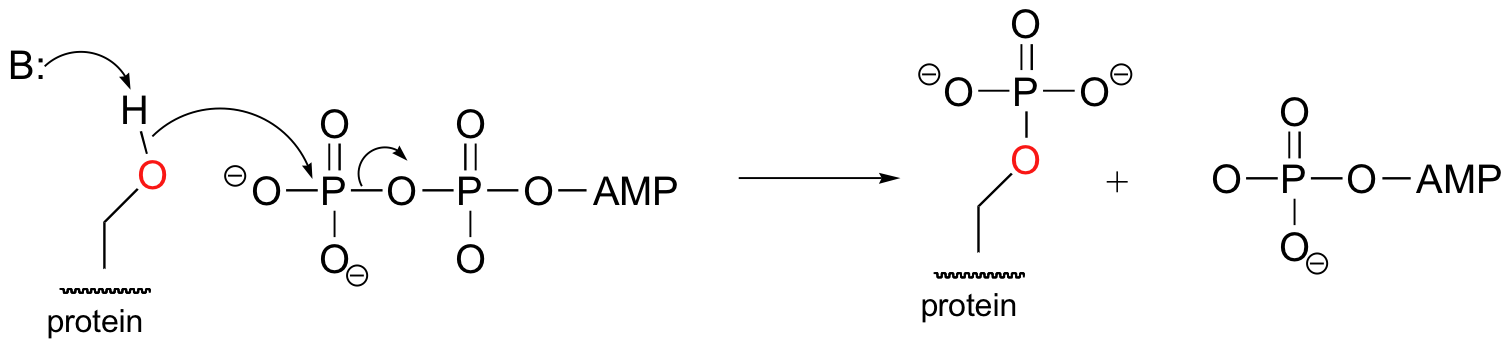
E10.4: The phosphorylation reaction does not involve any bonds to the two carbon stereocenters, so we do not expect any changes to stereochemical configuration.
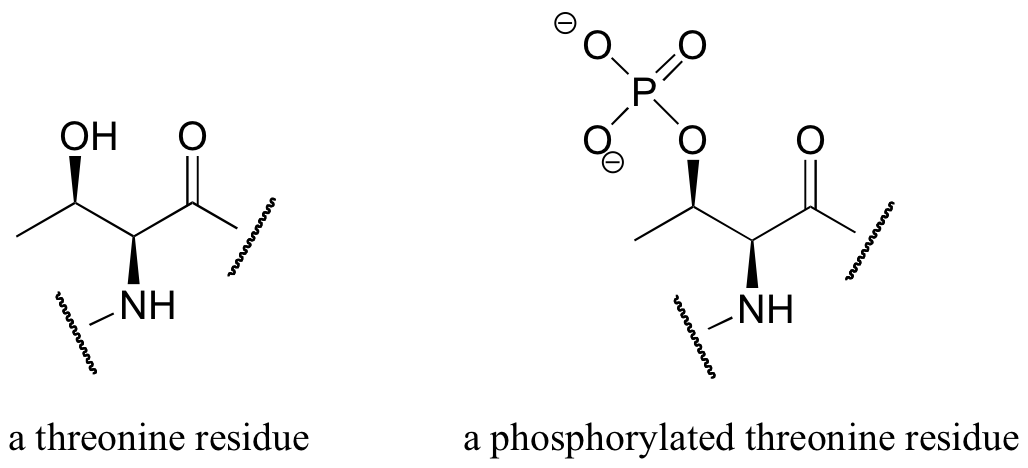
E10.5:
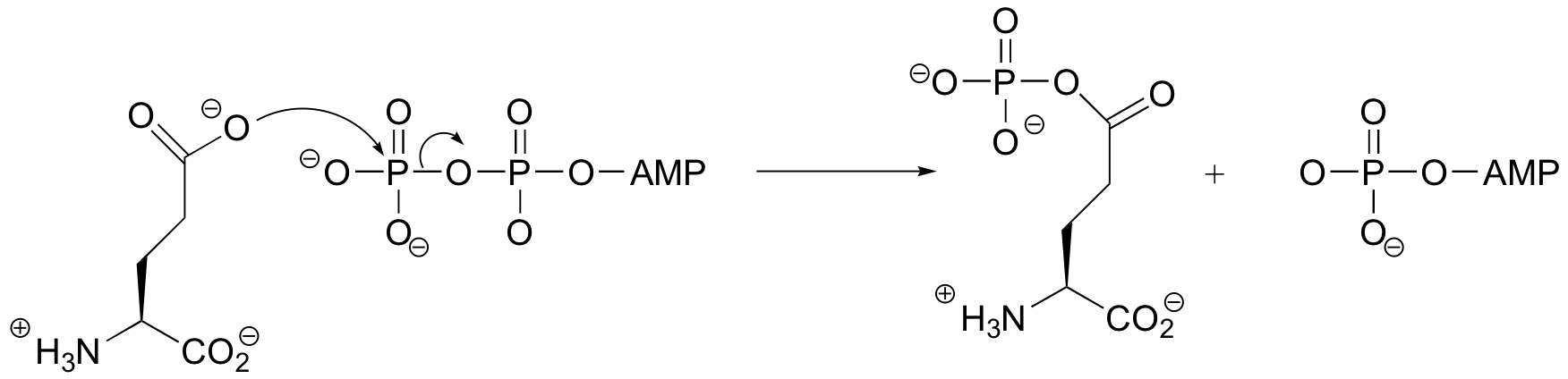
End-of-chapter problems
P10.1
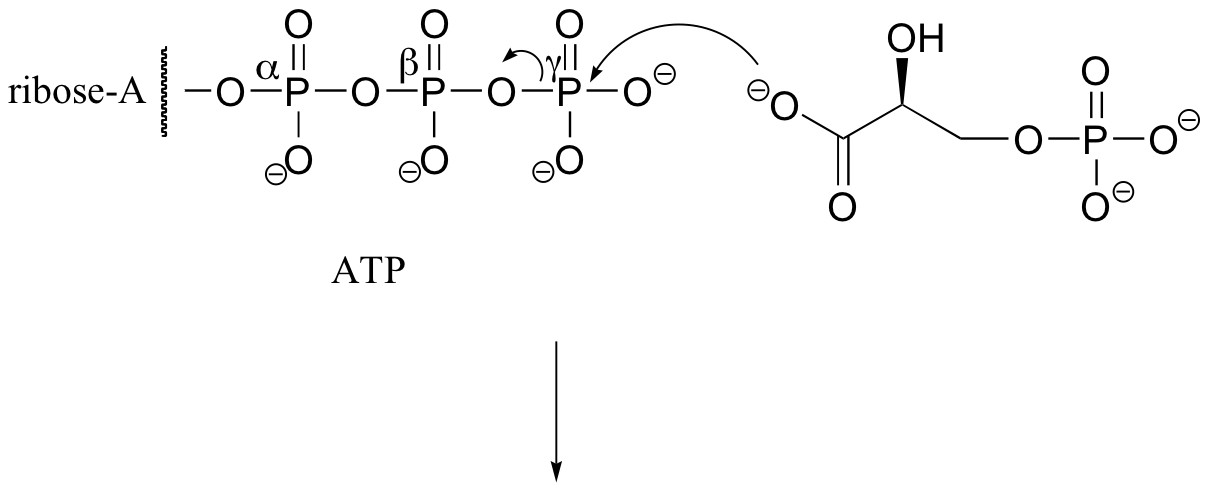
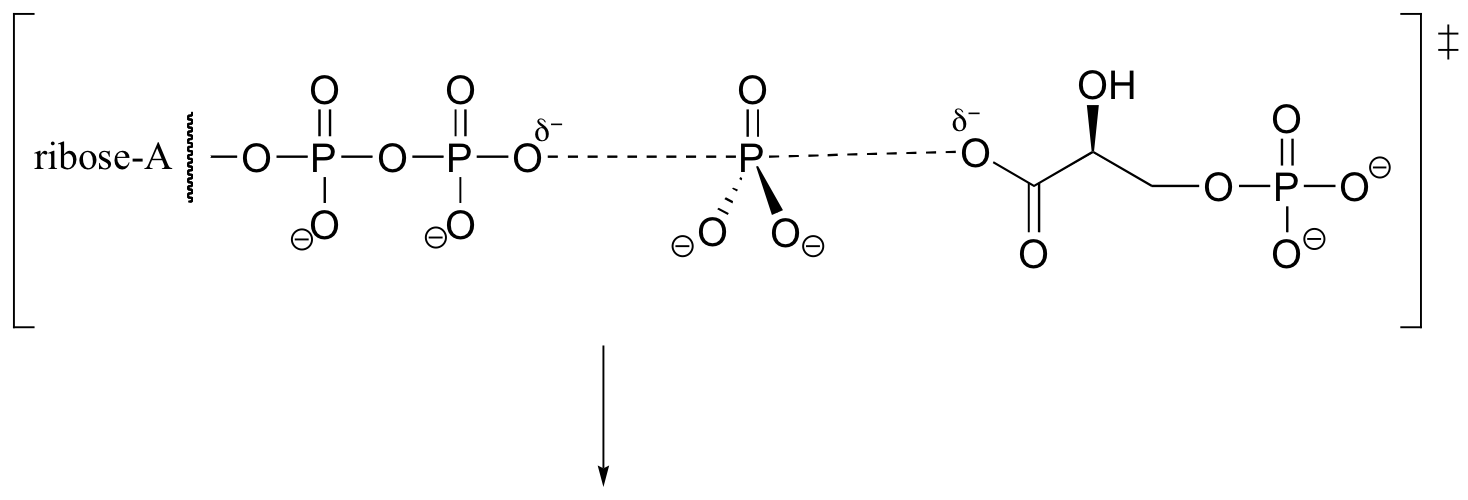
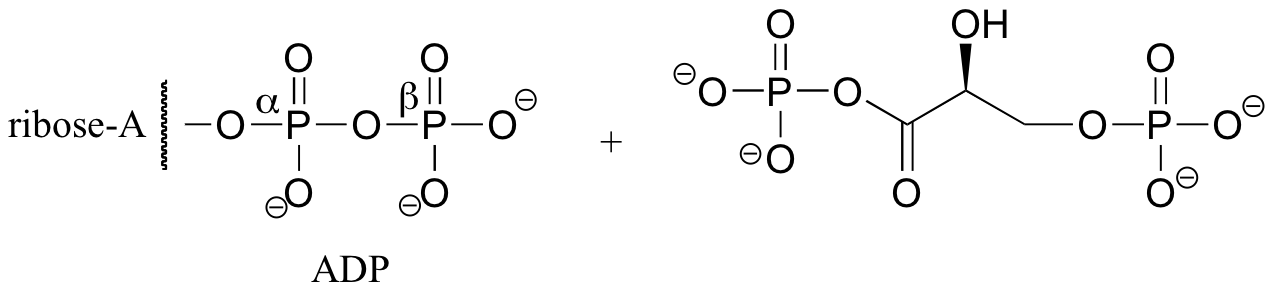
P10.2: Because no bonds to stereocenters are affected, the stereochemistry is unchanged.
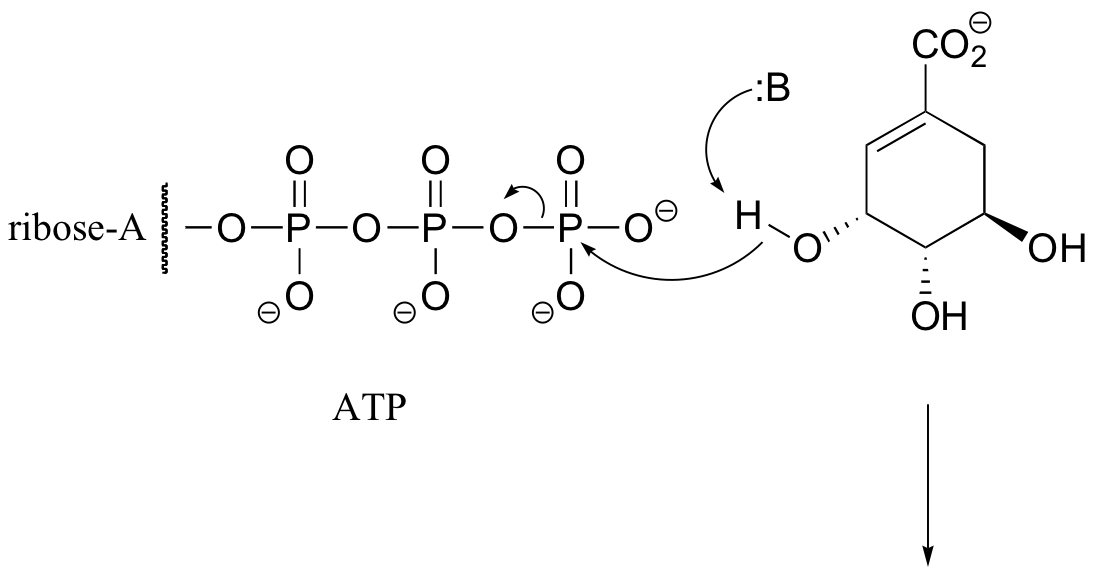
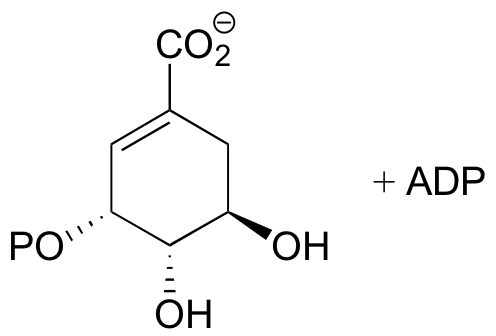
P10.3:
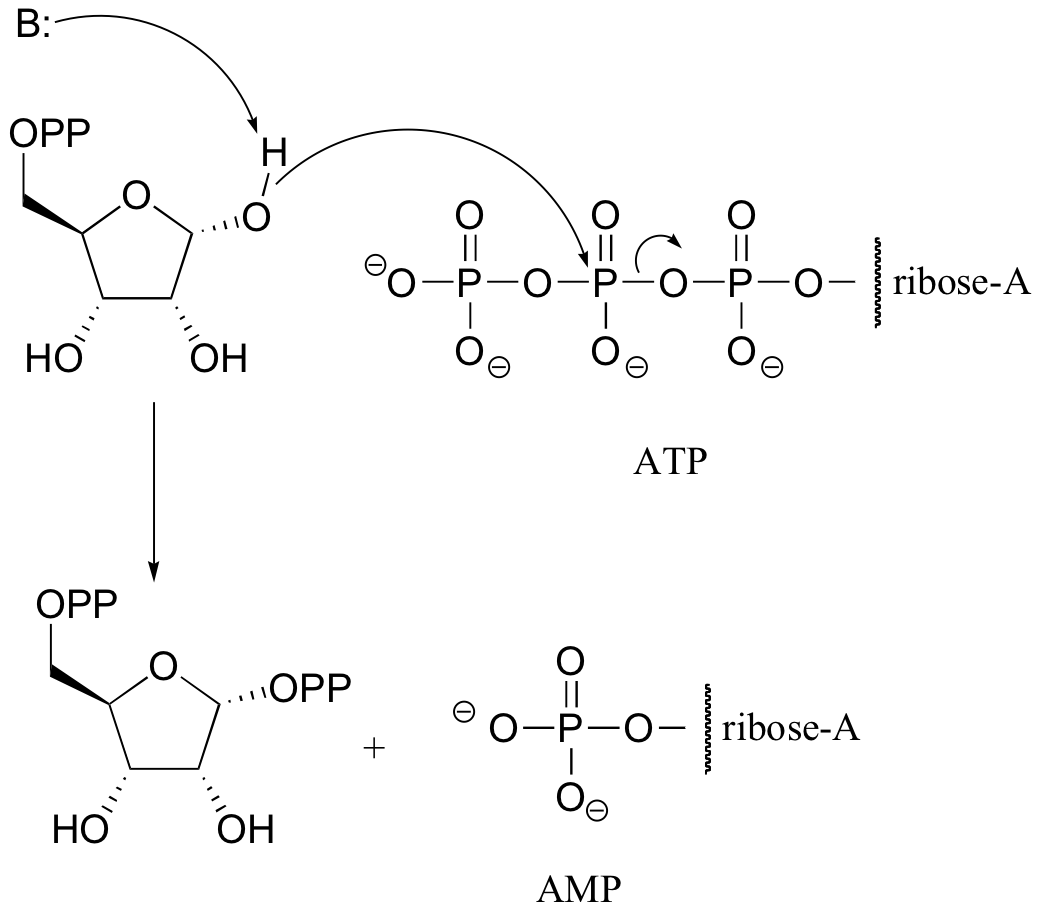
P10.4:
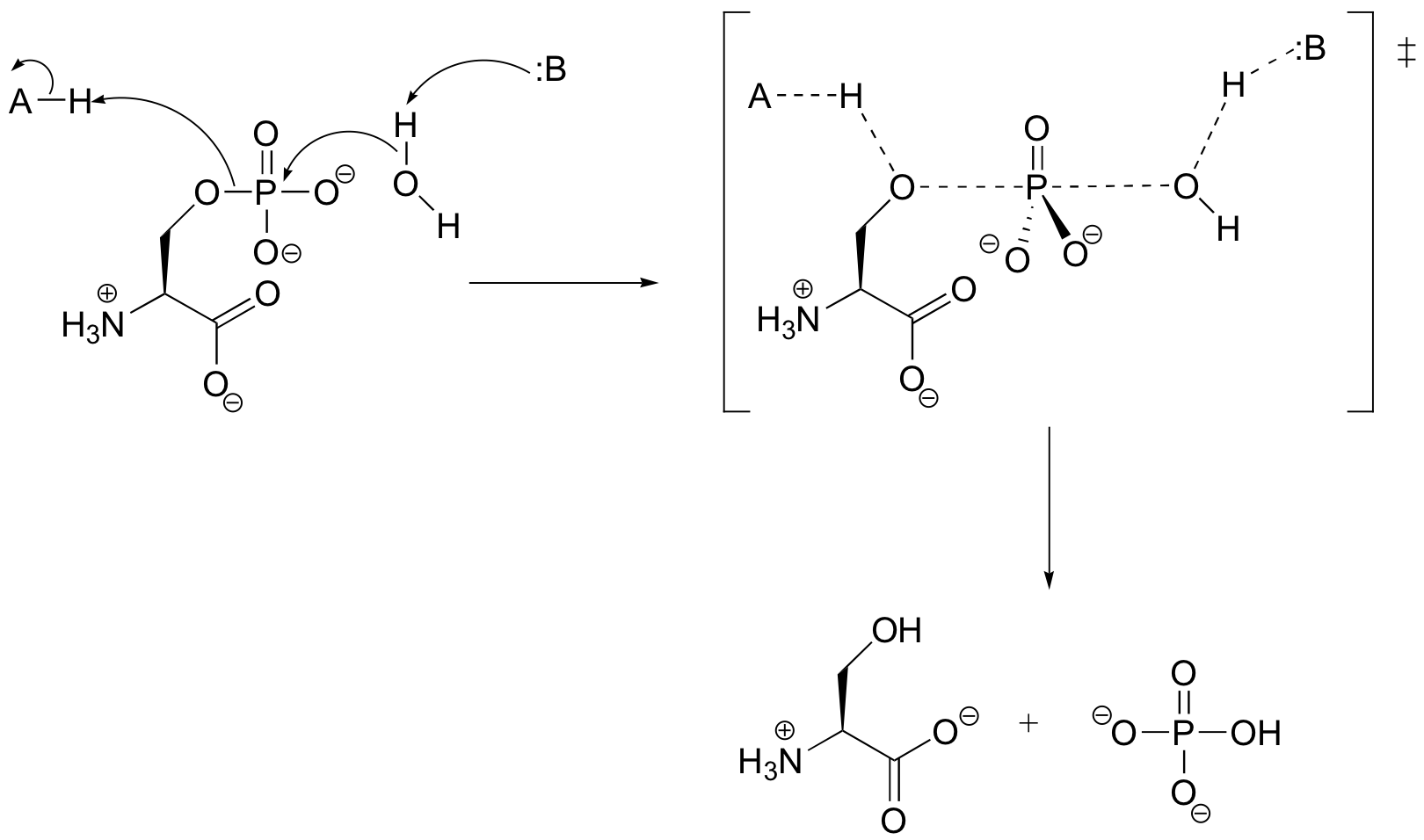
P10.5:
a)
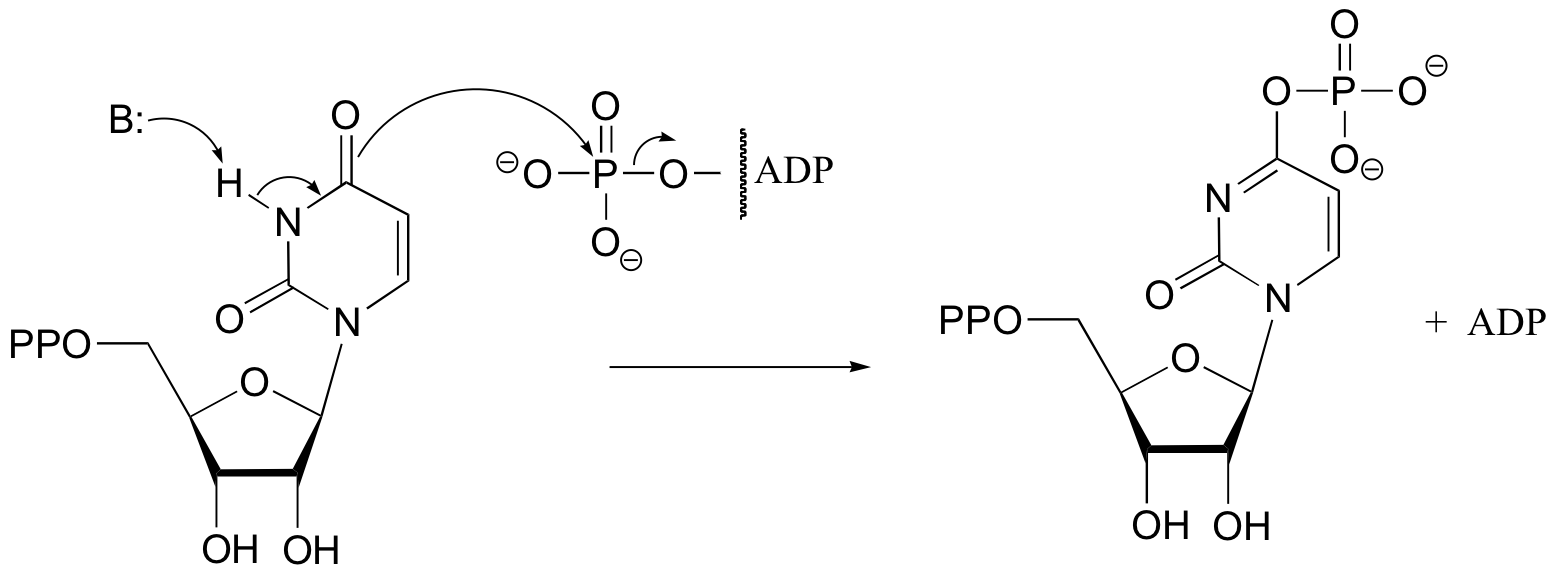
b)
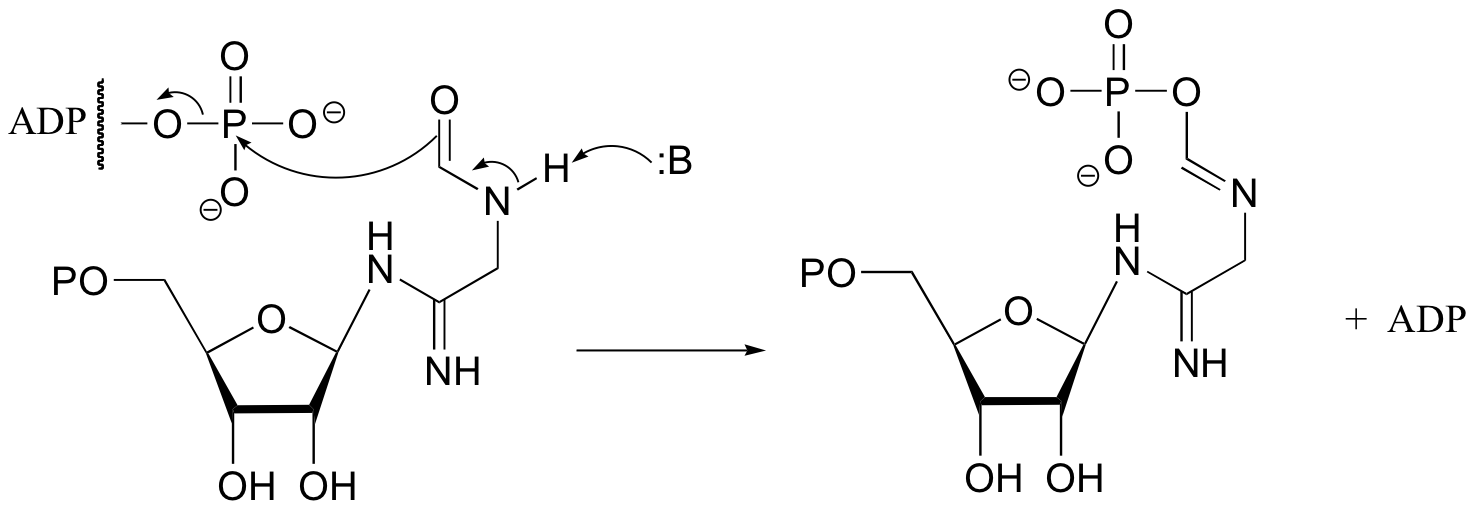
P10.6:
This is a transfer of a phosphate group to a nitrogen rather than to an oxygen, but the mechanism is analogous.
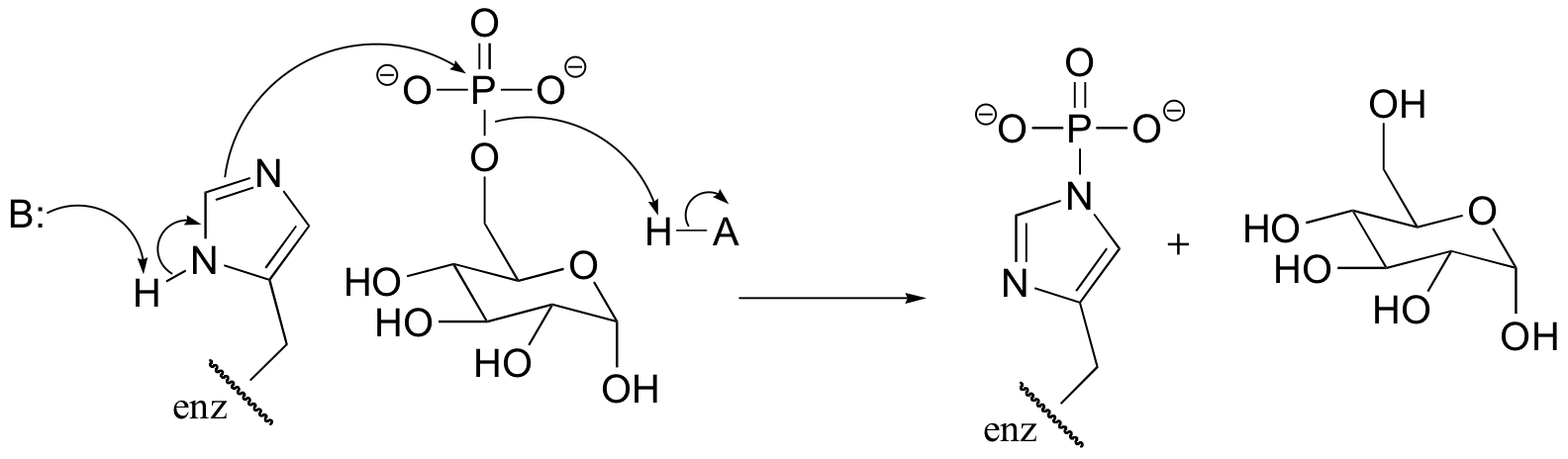
P10.7:
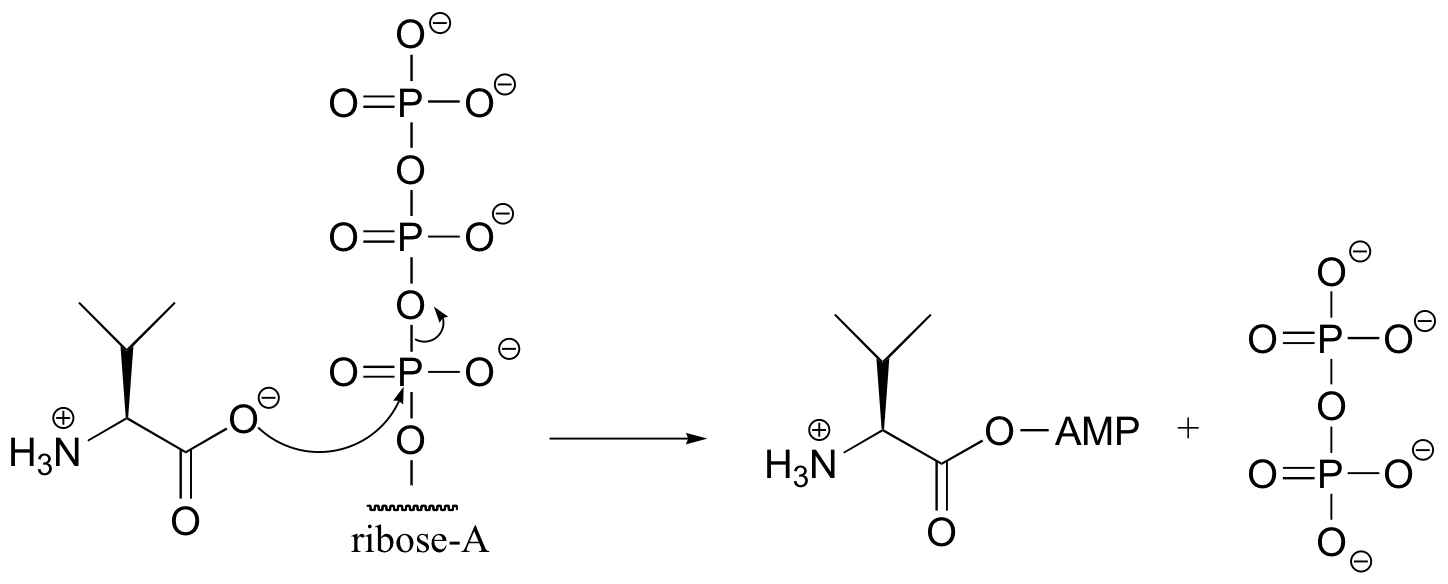
P10.8: (From J. Biol. Chem. 280, 10774)
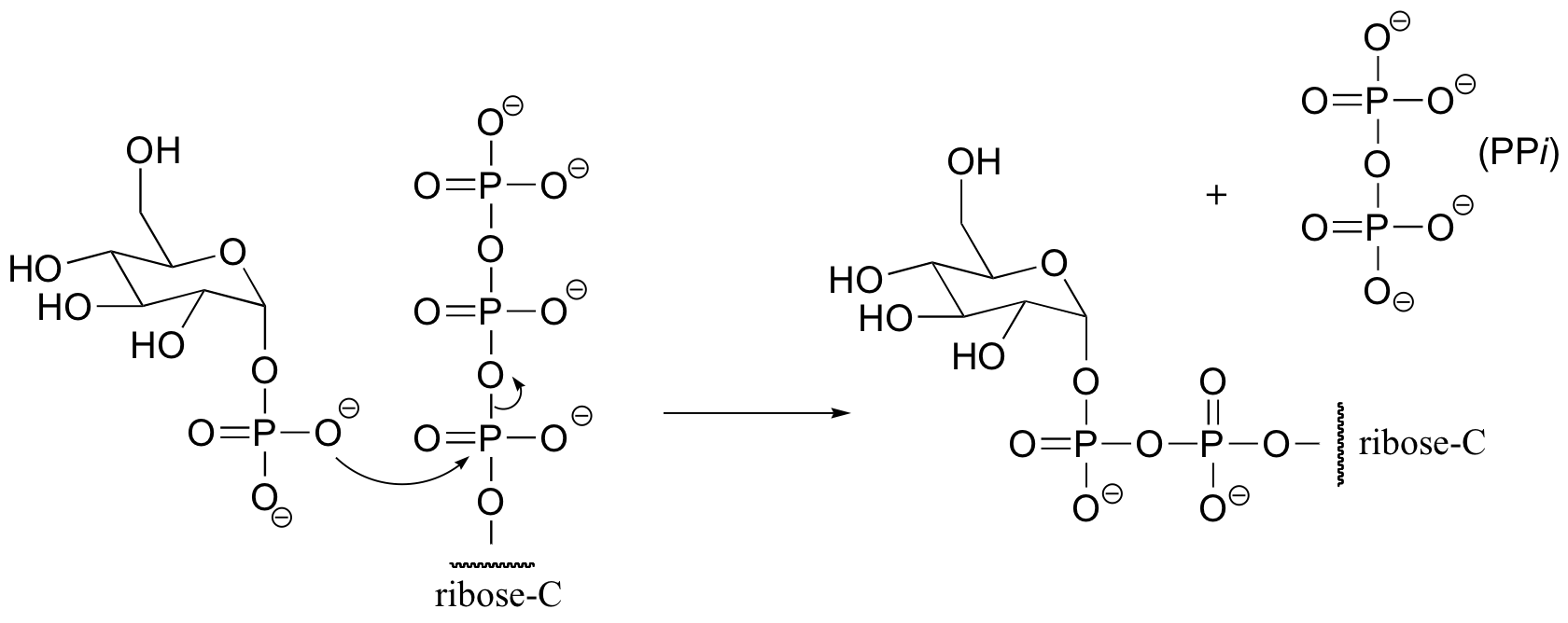
P10.9: (From Biochemistry 2000, 39, 8603)
a) The stereochemistry of the substitution and location of the 18O label in the product strongly suggests that this is an SN (probably SN1-like) displacement at the anomeric carbon atom, rather than attack by the water molecule at a phosphorus.


b) If water were to attack at the beta-phosphorus of the GDP group, the expected product would be:
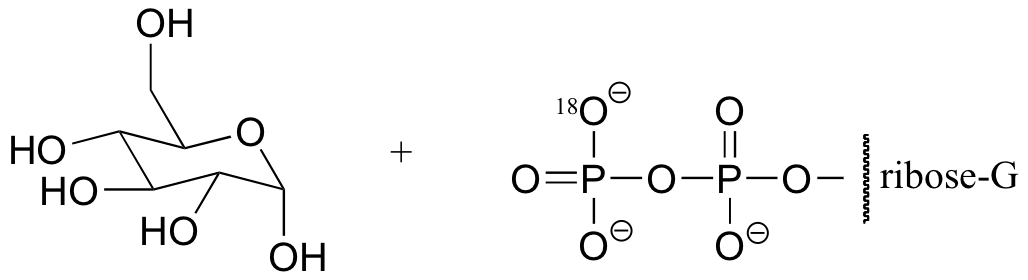
Notice the different stereochemistry at the anomeric carbon, and the different location of the 18O label.
P10.10: (From Biochemistry 44, 11476)
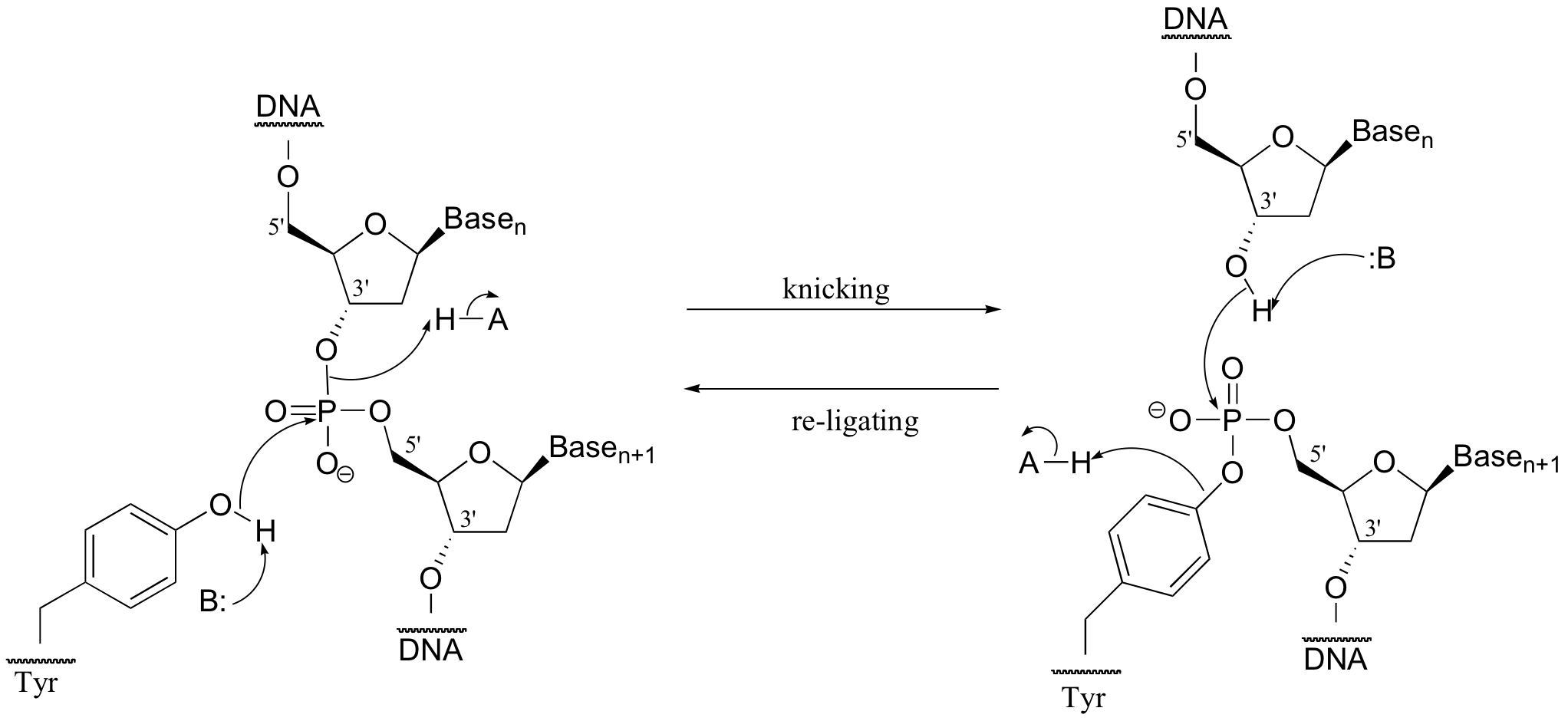
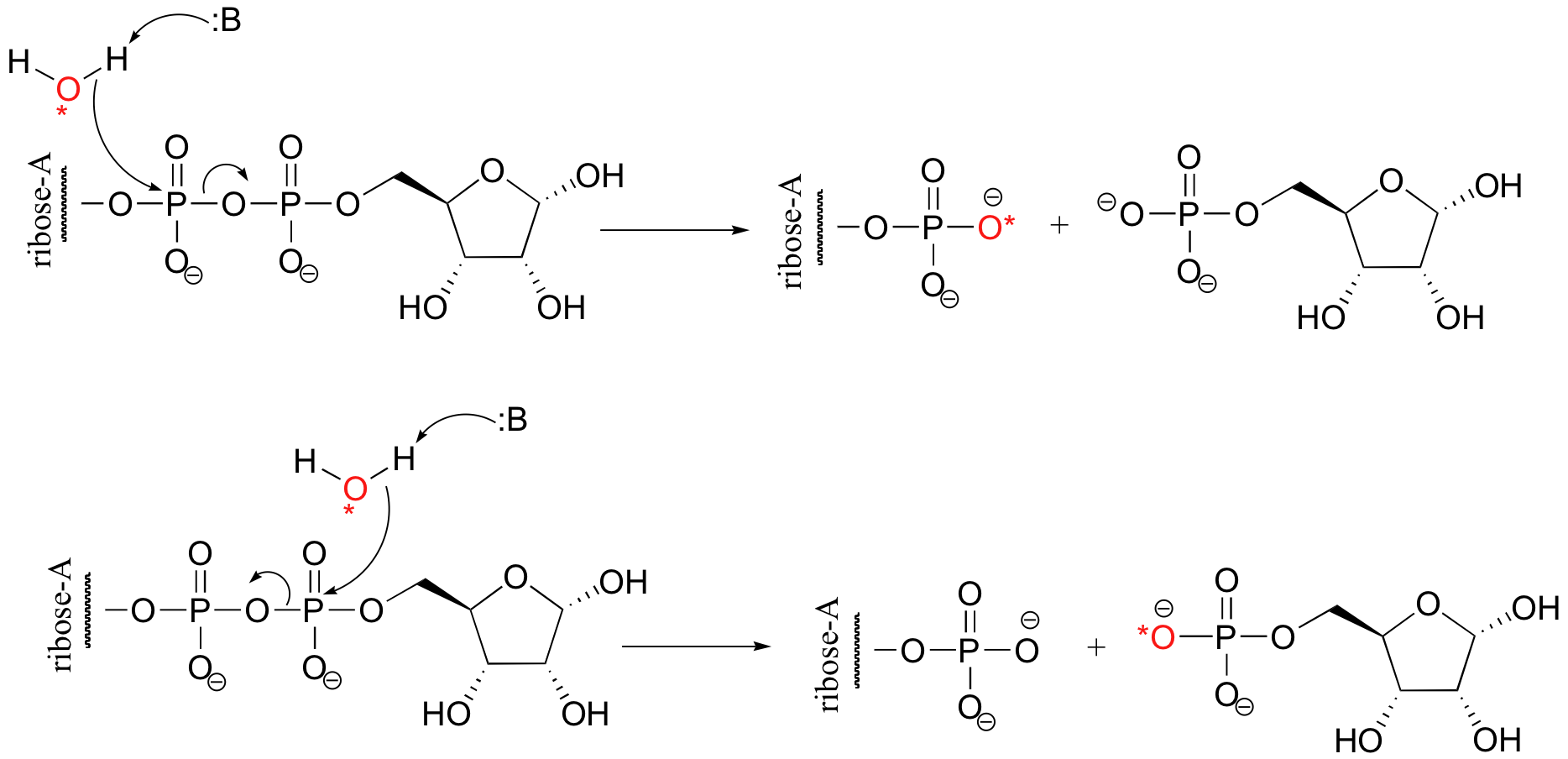
P10.11: (From Biochemistry 2002, 41, 9279). Using 18O-labelled water, we could determine the course of the reaction. In the first case, the AMP would contain the label, in the second case the sugar would contain the label. The authors of the study above, working with an enzyme from E. coli, found that the reaction proceeded by the top mechanism (attack by water at the adenosyl phosphate).
P10.12:
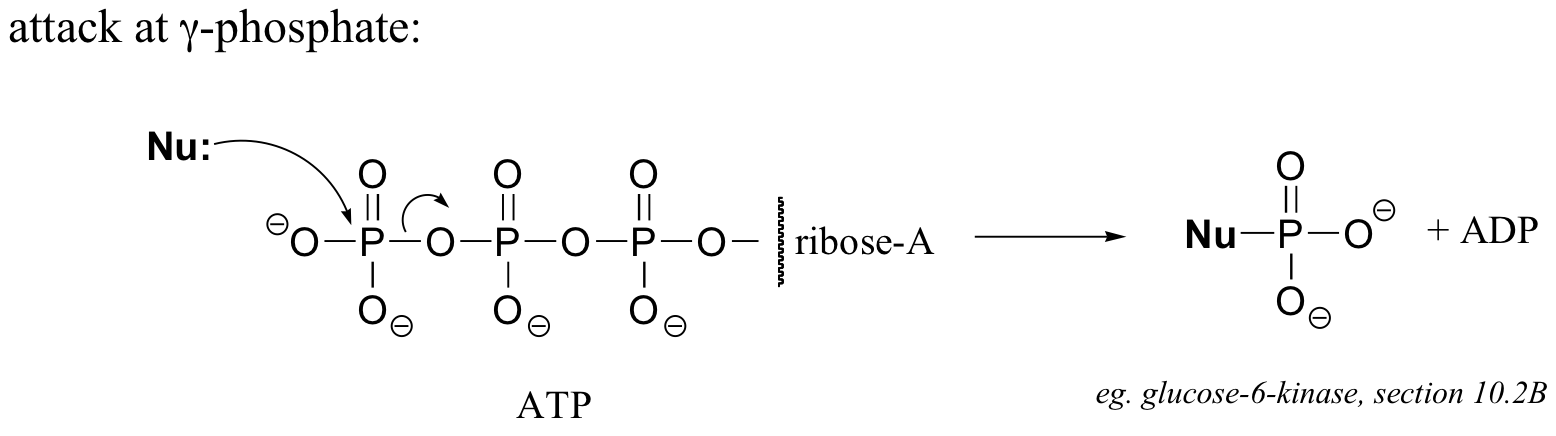

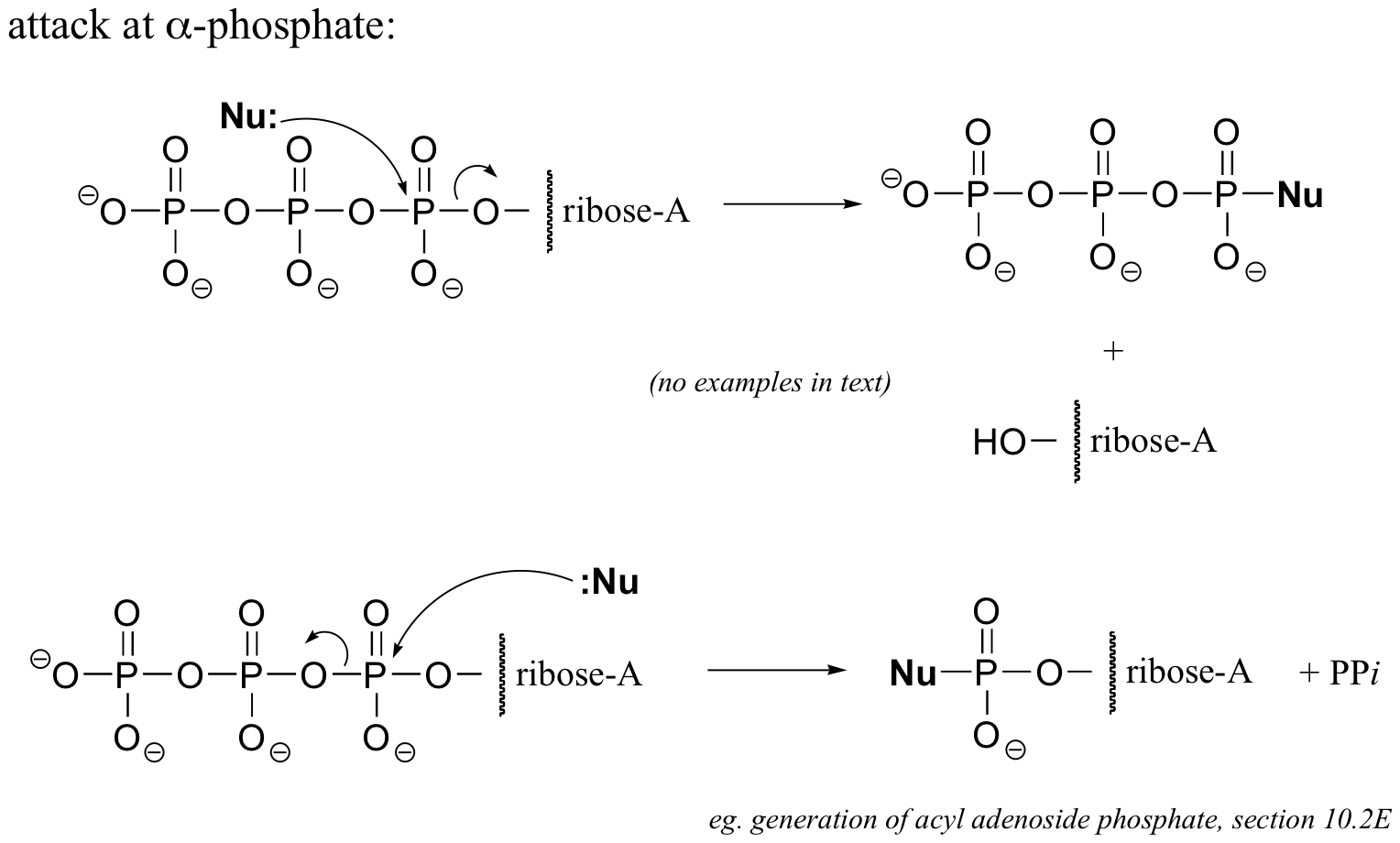
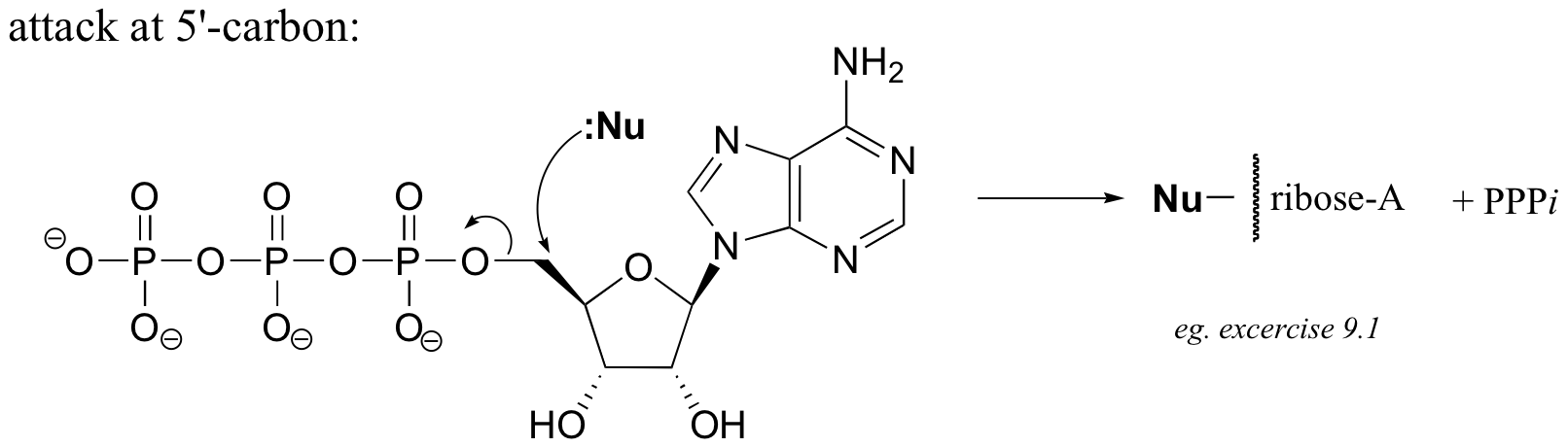
Here’s an example of this last possibility (the solution to exercise E9.1):
Challenge Problems
C10.1: See Biochem J. 1982, 201, 665.
C10.2: See J. Biol. Chem. 257, 14795.
C10.3: See J. Mol. Biol. 1999, 286, 1507.
Contributors
- Organic Chemistry With a Biological Emphasis, Tim Soderberg (University of Minnesota, Morris)


