Keck Asymmetric Allylation
- Page ID
- 92182
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Keck asymmetric allylation is an organic reaction in which an aldehyde can be converted to a homoallylic alcohol with high enantioselectivity. It is named after the University of Utah chemist Dr. Gary E. Keck. This reaction is also known as catalytic asymmetric allylation (CAA). The catalyst used in this reaction is a complex of (R) or (S)-BINOL (1,1'-Bi-2-naphthol) and Ti(OiPr)4. The catalyst has been given an acronym "BITIP" derived from the words BINOL and Titanium isopropoxide. There are 4 methods of preparation of the catalyst. They vary in the proportions of BINOL and Ti(OiPr)4 as well as catalytic amounts of CF3COOH (TFA).
- Method A: BINOL and Ti(OiPr)4 (1:1), 4 Å MS (400 mg/mmol of aldehyde)
- Method B: BINOL and Ti(OiPr)4 (2:1), 4 Å MS (400 mg/mmol of aldehyde), TFA (0.003 equivalent with respect to the aldehyde)
- Method C: BINOL and Ti(OiPr)4 (2:1), 4 Å MS (400 mg/mmol of aldehyde)
- Method D: BINOL and Ti(OiPr)4 (2:1)
Example reaction
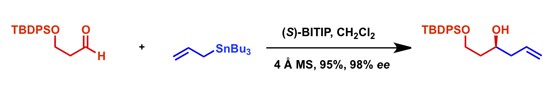
Mechanism
The mechanism of the reaction is not understood properly due the complex nature of the solution structure of BINOL-Ti co-ordination. It is also important to note that the experimental techniques to probe the mechanism seemed to be hopelessly slow when compared to the chemical events in the reaction.
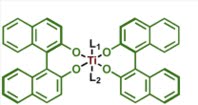
It is assumed that with 2:1 ratio of BINOL and Ti(OiPr)4, the solution structure of the catalyst most likely is (BINOL)2Ti, although other co-ordination structures are also possible. Keck and co-workers found that with Furfural this reaction (method A) yields product with 88% ee even with BITIP catalyst of 50% ee. This indicates a positive non-linear effect which implies the reactivity of the meso BITIP complex is less than that of the chiral catalyst. A simple structure of the meso complex is shown in the left figure.
E. J. Corey proposed a transition state structure of the catalyst (figure at the right) bound to the aldehyde where he invoked the idea of formyl C-H---O hydrogen bonding to
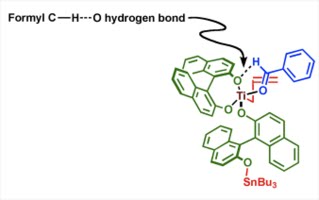
explain the observed absolute stereochemistry. Based on some crystal structure studies done by Reetz and Corey, it was proposed that the C-H bond length in this special "co-operative hydrogen bonding" should be less the sum of the Van der Waals radii of the H atom (1.20 A) and the O atom (1.52 A). This formyl hydrogen bonding is only possible if there is a strong co-ordination of the aldehyde carbonyl oxygen with the chiral Lewis acid. He also argued further that the aldehyde if switches position with the allyl group will lose the formyl C-H---O hydrogen bonding due to the steric hindrance provided by one of the BINOL ligand. Also positioning aldehyde and allyl group both at the basal position will not be able to find the formyl hydrogen bonding. Note that transmetallation of the allylstannane with the Titanium species is another key hypothesis in this model.
As there are not enough direct proofs of this formyl hydrogen bonding apart from the crystal structures, this stereochemical model is still not widely accepted to explain the absolute stereochemistry.
Role of Molecular sieves
Interestingly, molecular sieves used in this reaction is probably serving the purpose of providing some water molecules in the reaction mixture (except method D). Mikami and co-workers proposed that the active catalyst can only be formed due the presence of water molecules in the molecular sieves. This indicates the water molecules are probably acting as acid/base catalyst. Without the molecular sieves, lower yield of the reaction was observed. From the studies done by Keck and co-workers, the role of the molecular sieves seems to be more complex than just providing water molecules or sequestering MeOH (when B(OMe)3 is added as an additive). Molecular sieves are also necessary for higher enantioselectivity.
Role of TFA
Catalytic amount of trifluoroacetic acid or even triflic acid is used in this reaction (only in method B). Proton sources play an important role in the formation of the active catalyst by protonating the isopropoxide groups bound to titanium and thereby making the Ti-O bond more labile.
Role of additives
Sometimes it is preferable to use some additives such as iPrsBEt2 or B(OMe)3 to increase the rate of the reaction as this reaction ideally takes about 3-7 days to go to completion. The role of B(OMe)3 was studied by Keck and co-workers. B(OMe)3 probably binds with free isopropanol molecules in the reaction mixture and the liberated MeOH from the borate esters is sequestered by the molecular sieves, thereby facilitating the activity of the catalyst which in turn increases the rate and yield of the reaction.
Applications
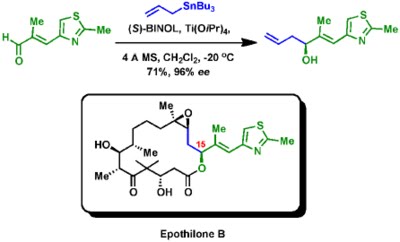
1. Keck and co-workers used this reaction in their elegant synthesis of the antitumor natural product epothilone B. The C15 stereocenter was set up using the CAA reaction.
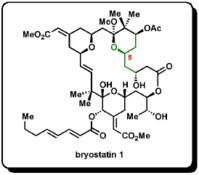
2. The example reaction is taken from the synthesis of bryostatin 1 by Keck and co-workers. The C5 stereocenter was established with CAA. Later on, this stereocenter was used for the stereochemical induction on C3 and C7 carbons. The structure of bryostatin 1 is given below:
References
For the seminal publications on CAA, see:
- 1. Keck, G. E.; Tarbet, K. H.; Geraci, L. S. J. Am. Chem. Soc. 1993, 115, 8467-8468.
- 2. Keck, G. E.; Geraci, L. S. Tetrahedron Lett. 1993, 49, 7827-7828.
- 3. Keck, G. E.; Krishnamurthy, D.; Grier, M. C. J. Org. Chem. 1993, 58, 6543-6544. (discussion on non-linear effect)
- 4. Keck, G. E.; Krishnamurthy, D.; Roush, W. R.; Reilly, M. L. Org. Synth. 1998, 75, 12.
- For the transition state model by Corey, see:
- 4. Corey, E. J.; Lee, T. W. Chem. Commun. 2001, 1321-1329.
- For the role of molecular sieves and additives, see:
- 5. Mikami, K. Pure & Appl. Chem. 1996, 3, 639-644.
- 6. Kurosu, M.; Lorca, M. Synlett 2005, 7, 1109-1112.
- 7. Heumann, L. V.; Keck, G. E. Org. Lett. 2007, 21, 4275-4278.
- For related applications of the catalyst, see:
- 8. Keck, G. E.; Krishnamurthy, D. J. Org. Chem. 1996, 61, 7638-7639.
- 9. Professor Douglass Taber's article in Organic chemistry portal: http://www.organic-chemistry.org/Highlights/2009/06July.shtm (synthesis of epothilone B)
- 10. Keck, G. E.; Giles, R. L.; Cee, V. J.; Wager, C. A.; Yu, T.; Kraft, M. B. J. Org. Chem. 2008, 24, 9675-9691. (synthesis of epothilone B)
- 11. Keck, G. E.; Poudel, Y. B.; Cummins, T. J.; Rudra, A.; Covel, J. A. J. Am. Chem. Soc. 2011, 4, 744-747. (synthesis of bryostatin 1)

