Acetoacetic Ester Synthesis
- Page ID
- 112618
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Acetoacetic ester (ethyl acetoacetate) is an extremely useful molecule that can be used to make ketones and other molecules. You’ll even use this later on in amino acid synthesis, so let’s break down the way it reacts.
From Beta-Ketoester to Ketone:
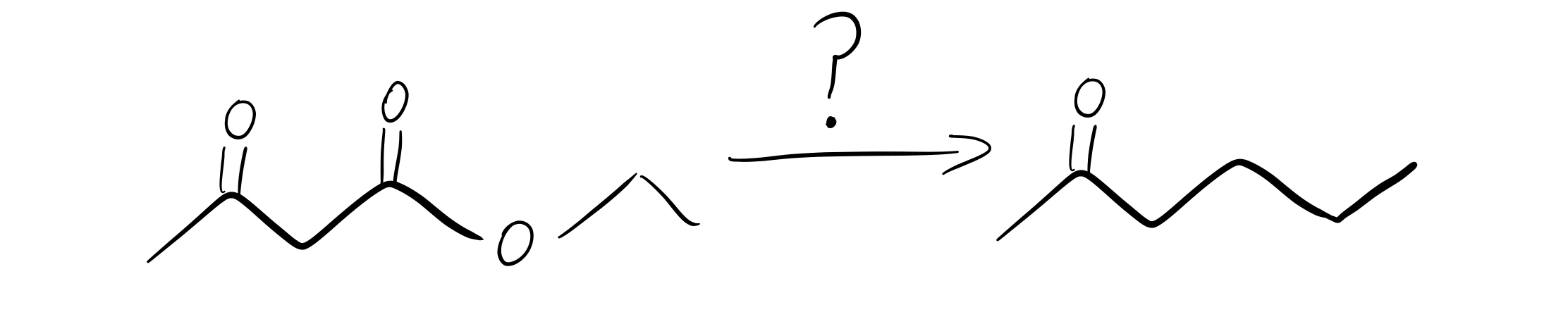 How do we accomplish this transformation
How do we accomplish this transformation
Enolate Formation
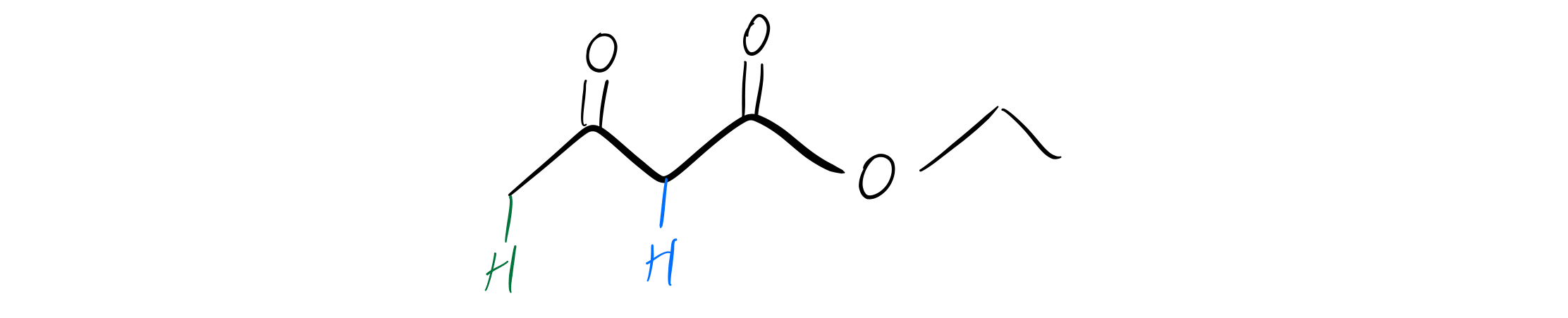
Labeled alpha-carbons
See those two carbonyls there? Each carbonyl has something called an alpha-carbon, and each alpha-carbon has hydrogens that are easily abstracted. The pKa of the green alpha-hydrogen is about 20, and the pKa of the blue alpha-hydrogen is actually about 10. Why? Because of the resonance structures the anions can form!
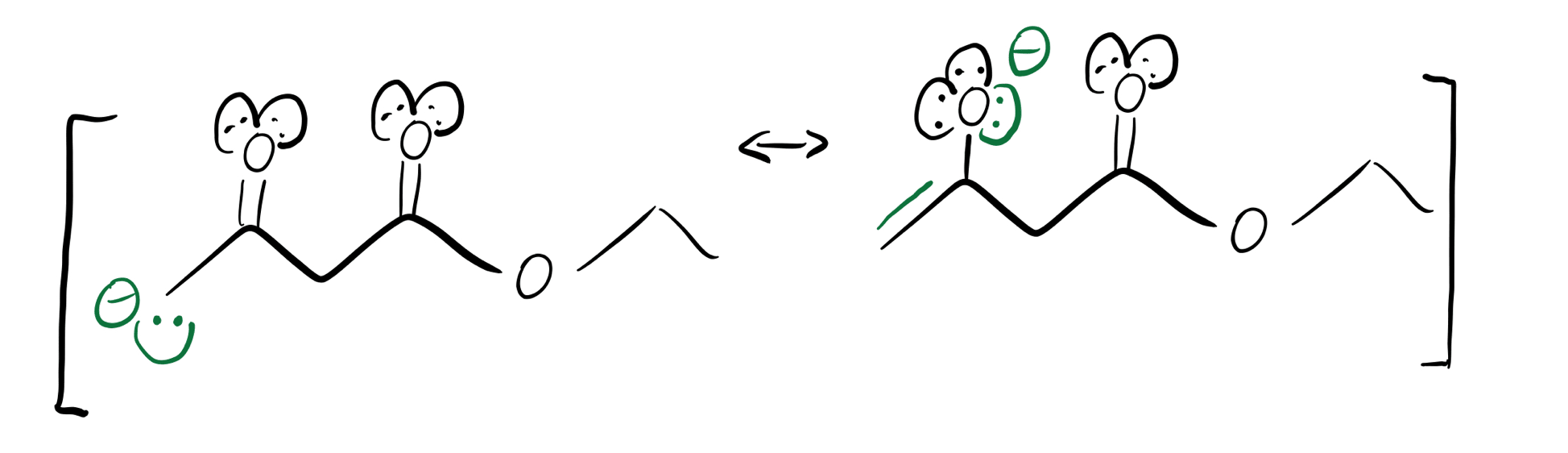 Green enolate resonance structures
Green enolate resonance structures
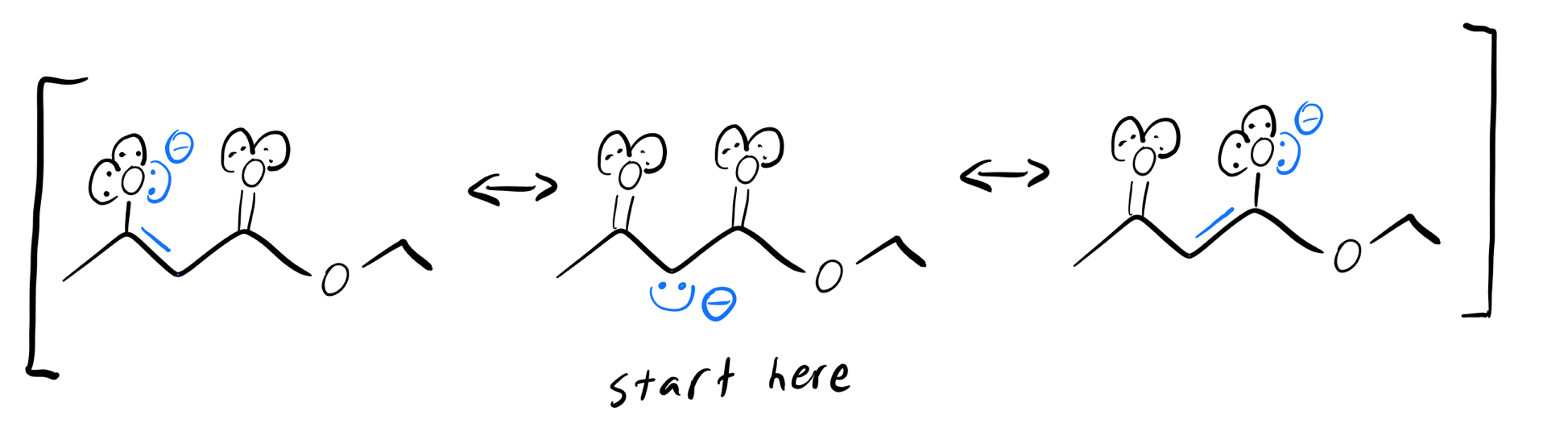 Blue enolate resonance structures
Blue enolate resonance structures
Whenever you have a beta-dicarbonyl like this one, the enolate will preferentially form on the shared alpha-carbon. The anion on the blue alpha-carbon above can form more resonance structures than the anion on the green alpha-carbon can, so the blue hydrogen’s pKa will be lower (more acidic).
That all sounds cool, but can we just use any ol’ base to form our enolate? Definitely not! Let’s say we were to try using NaOH. Instead of forming the enolate, we’d actually wind up with a competing reaction: saponification, a type of nucleophilic acyl substitution. Notice that the hydroxide replaces the ethoxy group.
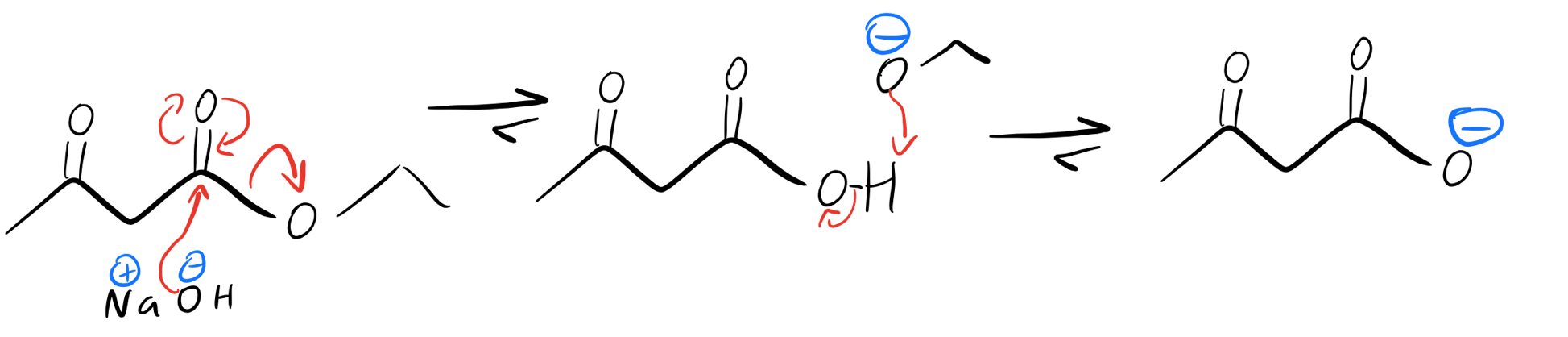 abridged saponification mechanism
abridged saponification mechanism
So, how can we specifically avoid that type of acyl substitution? We can use a bulky base like LDA or the anionic version of our alkoxy group! See how we’ve got an ethoxy group (—OEt) in our starting material? In order to prevent any substitutions of that group, we can actually use NaOEt. Those ethoxy groups are totally exchanging, but the same molecule is produced.
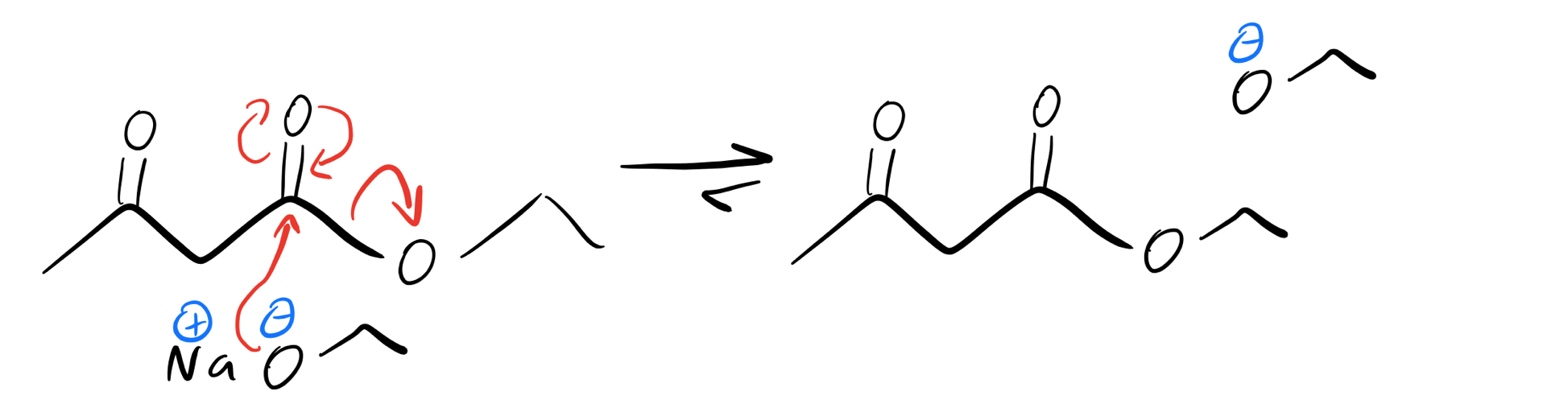 Fischer esterification with sodium ethoxide
Fischer esterification with sodium ethoxide
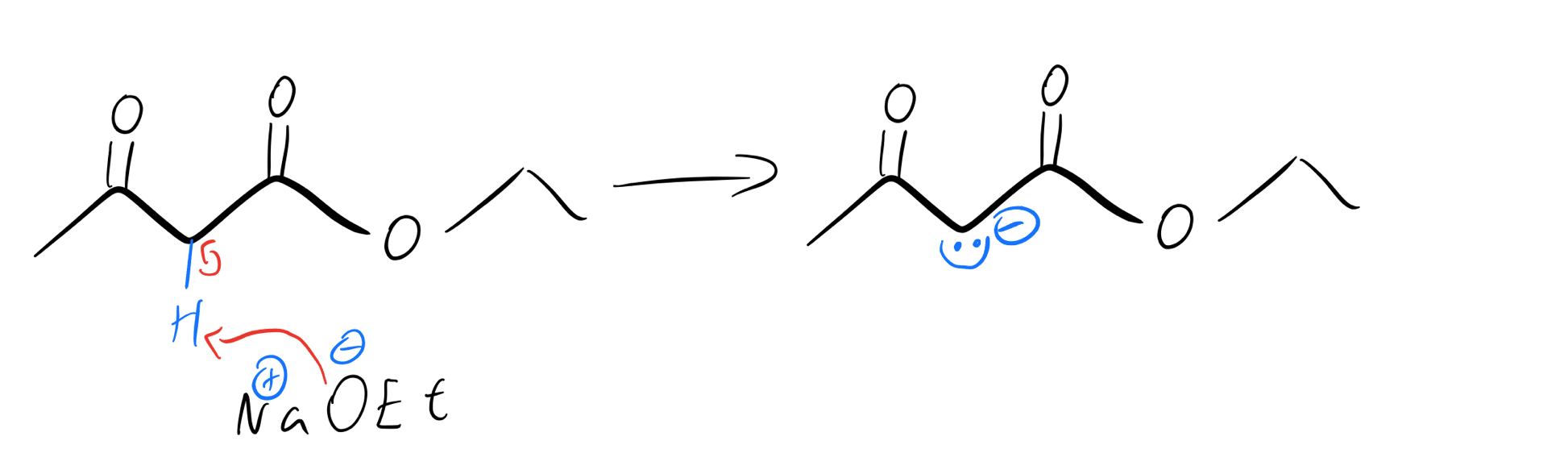 Methylene enolate formation
Methylene enolate formation
Enolate Alkylation
Okay, cool! These enolates are pretty good at SN2 reactions. They can act as nucleophiles on alkyl halides, acyl (acid) chlorides, and more! Let’s try adding a propyl group.
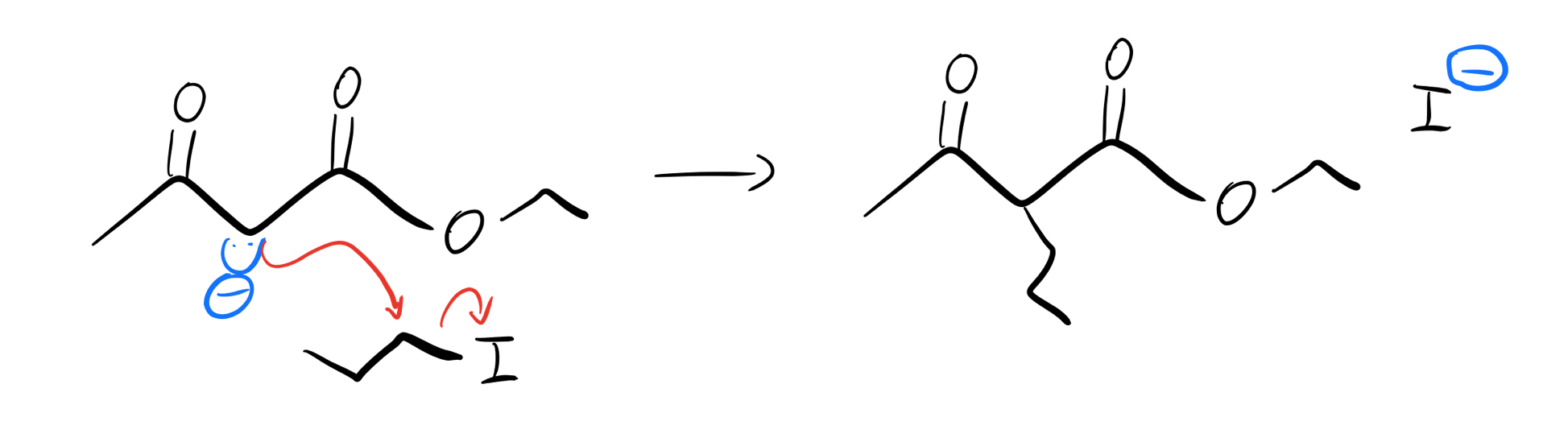 Enolate alkylation
Enolate alkylation
Decarboxylation
Once we’ve got our alkyl group on there, we can actually get rid of the ester entirely through a mechanism called decarboxylation if we want to. All it takes is some heat and a little bit of aqueous acid. It could be written a ton of different ways—H2SO4 (aq), HCl (aq), or even generically as H3O+.
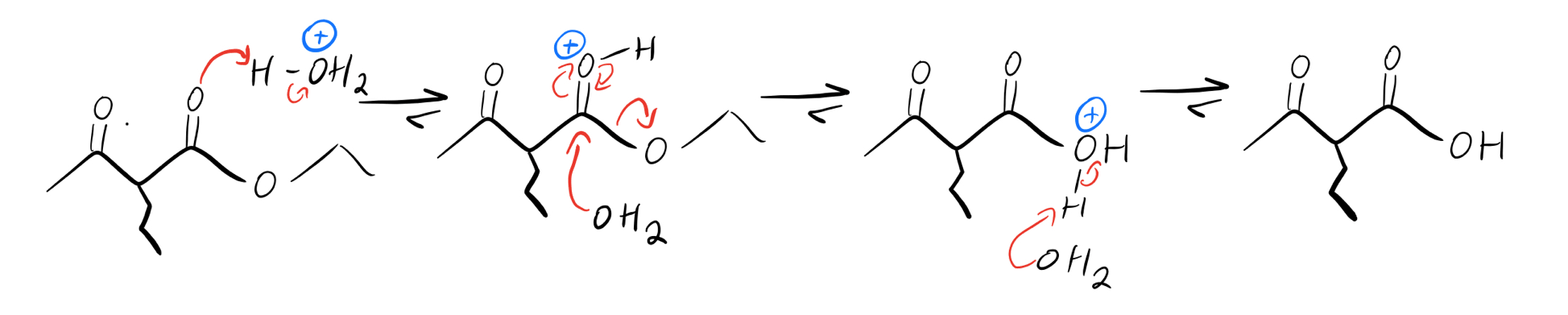 Acid-catalyzed ester hydrolysis
Acid-catalyzed ester hydrolysis
First we hydrolyze the ester to make a beta ketoacid, and then we heat things up to lose CO2. After acidic hydrolysis, the enol (vinyl alcohol) that results will tautomerize back into a substituted ketone.
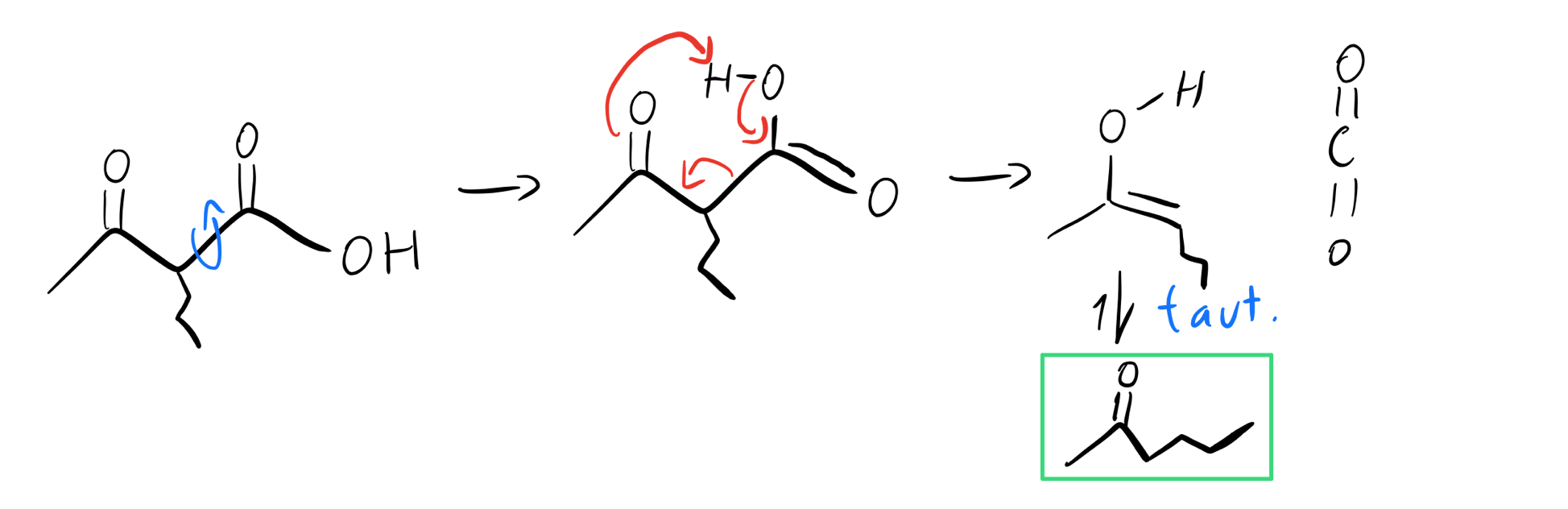 Tautomerization and decarboxylation
Tautomerization and decarboxylation
Boom! There’s our product, a substituted ketone, in the green box! Not so bad, right? Of course, there are tons of different ways to use this molecule. We’ve just walked through the steps for a single alkylation, but there’s nothing stopping us from adding different groups.
Adding Two Alkyl Groups
We’ve added one alkyl group, but what if we want to add another one? Well, we just have to follow the same steps! So, let’s start from the beginning. Let’s first add a propyl group and then an ethyl group. Once we’ve added the propyl group, all we need to do is add another equivalent of base and then the ethyl group. Here’s what the order of reagents looks like:
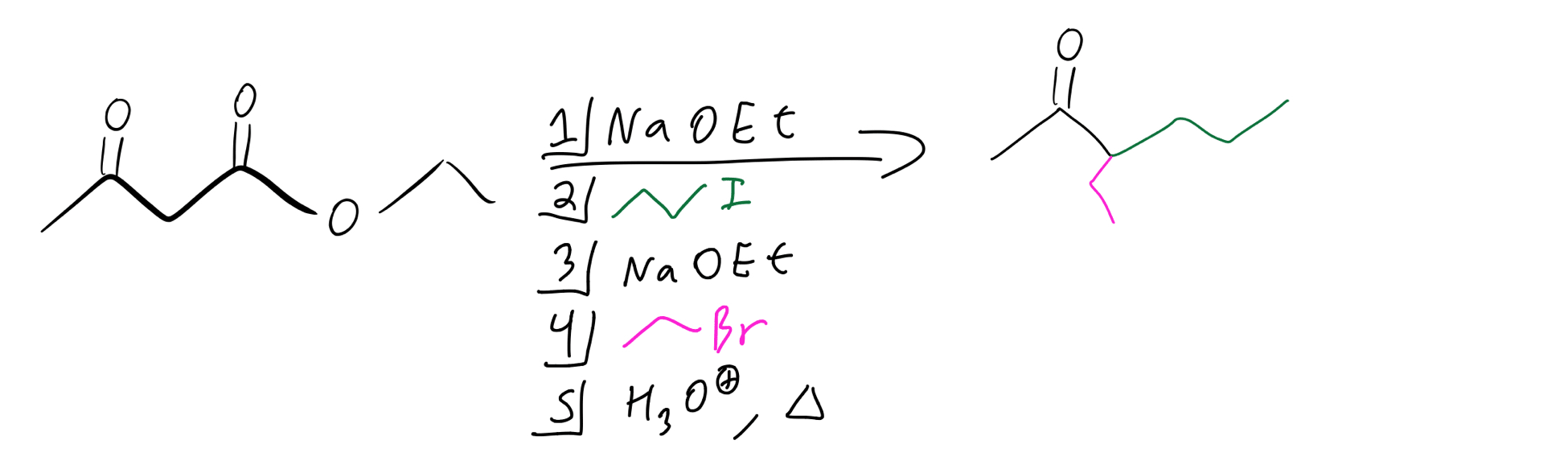 Double alkylation reagents
Double alkylation reagents
And here’s what the mechanism would look like:
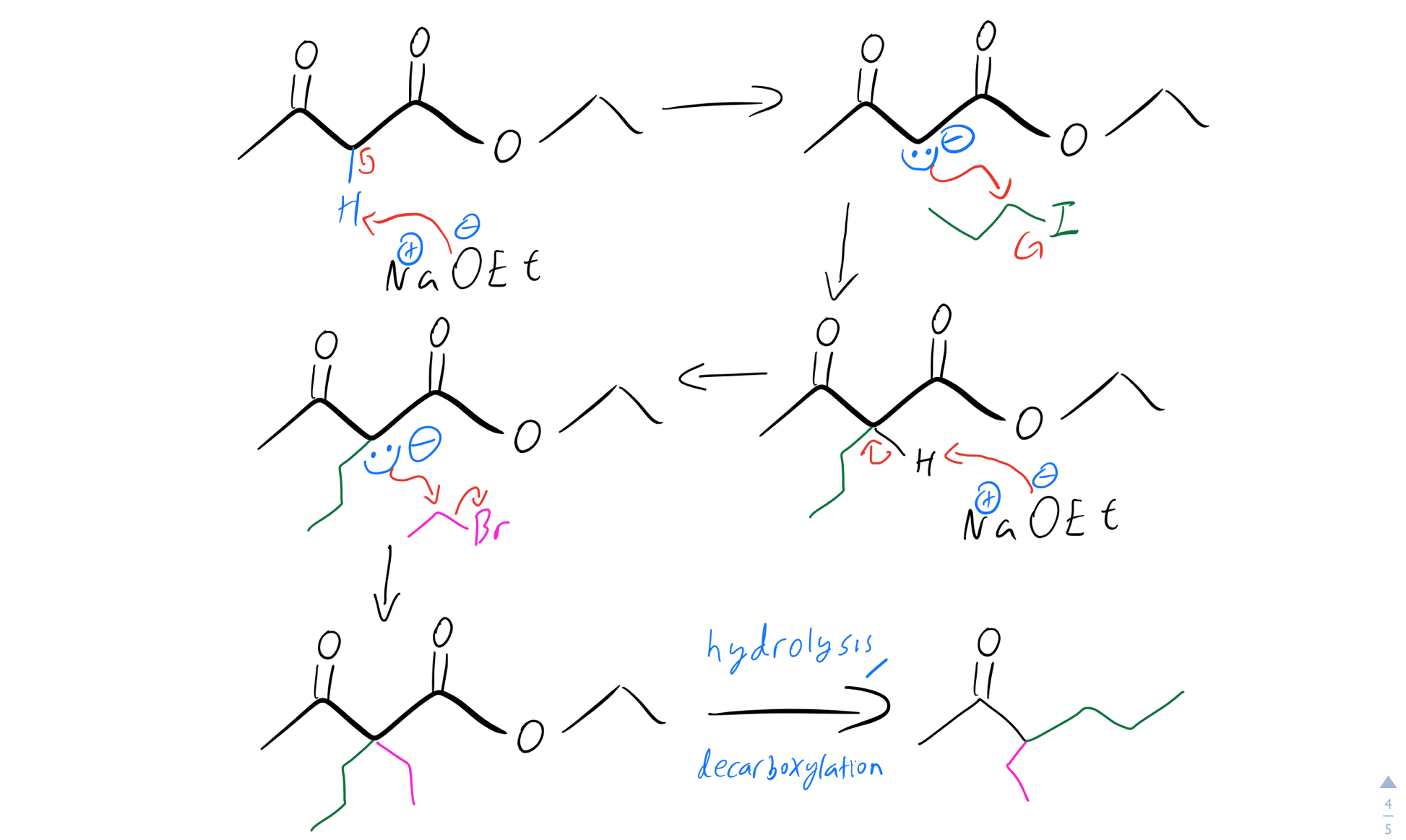 Double alkylation mechanism
Double alkylation mechanism
Adding Cyclic Alkyl Groups
Okay, but what if we want to add a cyclic group to our molecule? Well, luckily that’s not so bad either. We just need a molecule that has two leaving groups at terminal positions. Basically, it’s going to be very similar to the double alkylation but with just one equivalent of our alkyl molecule being added. Here’s what the reagents look like: 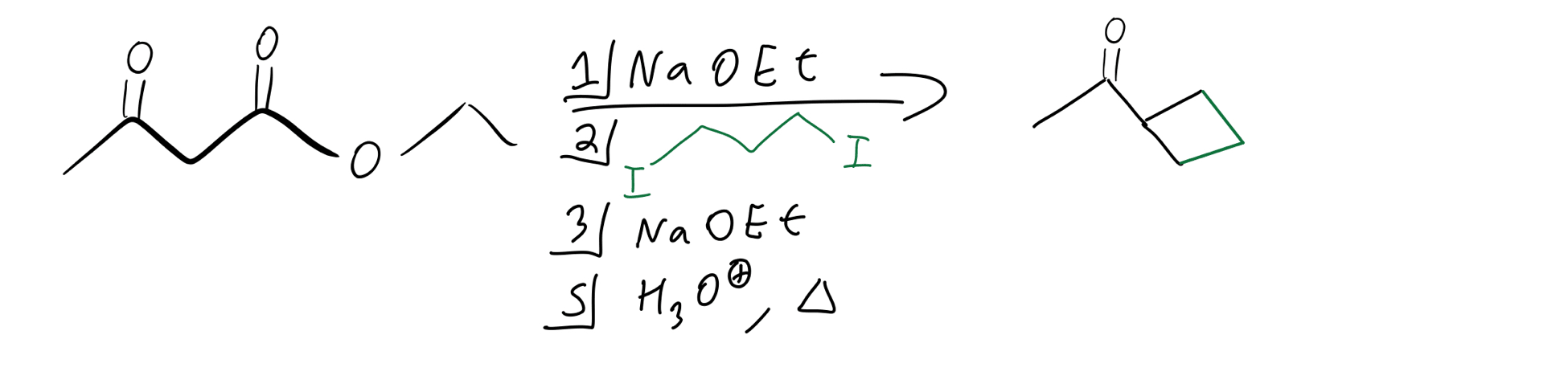
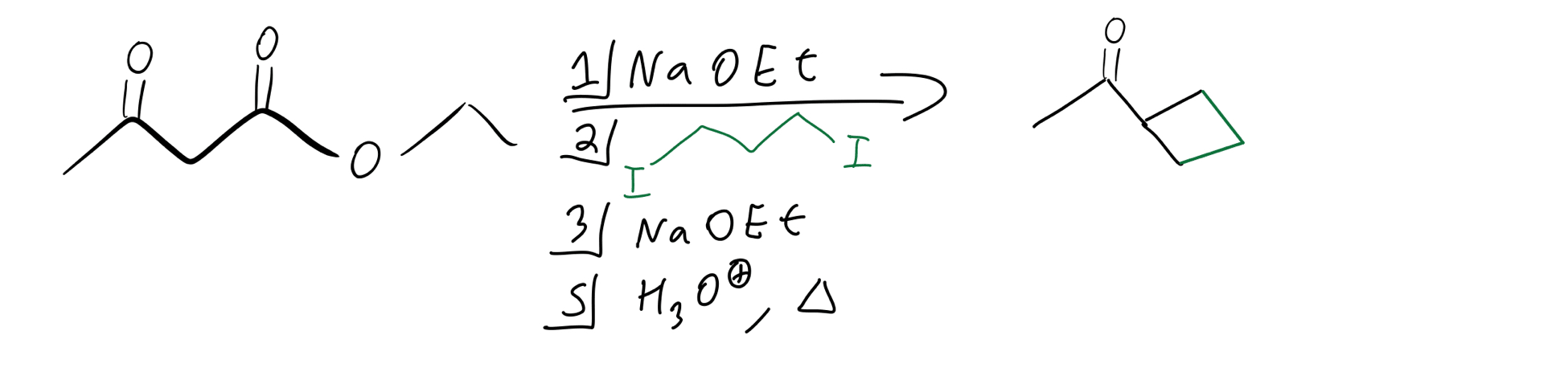 Cyclic alkylation reagents
Cyclic alkylation reagents
And here’s what the mechanism looks like:
 Cyclic alkylation mechanism
Cyclic alkylation mechanism
Adding Acyl Groups
Let’s take a step back and use the same enolate we used in the alkylation, but let’s use an acyl chloride instead of an alkyl halide this time. This follows basically the same pattern as the alkylation, but I’m going to rotate the acetoacetic ester a little bit and highlight the acid chloride so that it’s easier to follow.
 Enolate acylation mechanism
Enolate acylation mechanism
See how we just followed the same pattern? Form the enolate, provide an electrophile, and cleave off the ester by adding acid and heating it up!
Contributors
- Johnny Betancourt, Clutchprep. Source page can be accessed here.


