Basics of Catalysts
- Page ID
- 39040
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- What are chemical absorptions and how do they promote chemical reactions?
- What types of chemisorption lead to the poisoning of a catalyst?
- How transition metals are chosen as catalysts?
- What are syngases and how are they prepared?
- Why metal clusters will be excellent potential catalysts?
- Are non-stoichiometric oxides potential catalysts for redox reactions?
- What type of catalysts can be made of stoichiometric oxides?
- What are photocatalytic reactions?
Heterogeneous Catalysts
A catalyst is another substance than reactants products added to a reaction system to alter the speed of a chemical reaction approaching a chemical equilibrium. It interacts with the reactants in a cyclic manner promoting perhaps many reactions at the atomic or molecular level, but it is not consumed. Another reason for using a catalyst is that it promote the production of a selected product.
A catalyst changes the activation energy, Ea, of a reaction by providing an alternate pathway for the reaction. The rate and rate constant k of a reaction are related to Ea in the following ways:
rate = k * function of concentration
k = A exp (- Ea / R T)
where A is a constant related to collision rates. Thus, a change in Ea changes the rate of a reaction.
A catalyst in the same phase (usually liquid or gas solution) as the reactants and products is called homogeneous catalyst.
A catalyst that is in a separate phase from the reactants is said to be a heterogeneous, or contact, catalyst. Contact catalysts are materials with the capability of adsorbing molecules of gases or liquids onto their surfaces. An example of heterogeneous catalysis is the use of finely divided platinum to catalyze the reaction of carbon monoxide with oxygen to form carbon dioxide. This reaction is used in catalytic converters mounted in automobiles to eliminate carbon monoxide from the exhaust gases.
Promoters are not catalysts by themselves but increase the effectiveness of a catalyst. For example, alumina Al2O3, is added to finely divided iron to increase the ability of the iron to catalyze the formation of ammonia from a mixture of nitrogen and hydrogen. A poison reduces the effectiveness of a catalyst. For example, lead compounds poison the ability of platinum as a catalyst. Thus, leaded gasoline shall not be used for automobils equiped with catalytic converters.
Because heterogeneous catalysts often are used in high temperatures reactions, they are usually high melting (refractory) materials, or else they can be supported by refractory materials such as alumina.
Today, catalysts design is a challenge for chemists and engineers for effective productions, pollution prevention, and waste treatments.
What are chemical absorptions and how do they promote chemical reactions?
As mentioned in solid defects, solid surfaces are two-dimensional defects. They offer a potential for attraction to molecules of gases and liquid. Adsorption takes place as molecules are attracted to the surface, and when molecules penetrate through the bulk material, the term absorption is used. Absorption with no chemical bonds formed or broken is called physical absorption or physisorption, whereas chemisorptions refer to processes when new bonds are formed or broken.
Inorganic Chemistry by Swaddle (page 117) gives an excellent example to illustrate the chemisorption of hydrogen by a nickel catalyst. The bond energy of H2 is 435 kJ/mol. Thus, in a hydrogenation reaction, energy has to be made available for the reactions:
H2 -> 2 H, H = 435 kJ
| |
>C=C< + 2 H -> H-C--C-H
| |
In the above reaction, the activation energy, Ea is close to 435 kJ. However, when hydrogen is absorbed by nickel, the breakage of the H-H bond is facilitated by a series of steps.
2 Ni + H2 --->2 Ni...H2----> 2 Ni-H
solid gas physisorption chemisorption
The activation energy is thus lowered due to the formation of Ni-H bonds. A change in activation energy changes the rate of reaction.
In the activation of O2 by a metal M, the O=O bond is weakend or broken via these steps:
O=O O--O O O O- O-
| | || || | |
-M--M- ==> -M---M- ==> -M M- ==> -M M-
In these steps, the oxygen is activated at various stages.
With sophisticated experimental techniques, we can study the chemisorbed species in details. For example, the chemisorbed ethylene is believed to be an ethylidyne radical
H H H
\ | /
C
|
C
/|\
PtPtPtPtPt
Metal Metal Metal
The chemisorbed ethylidune radical.
What types of chemisorption lead to the poisoning of a catalyst?
If the absorbed species are very stable, and much energies are release in the chemisorption process, the absorbed species are not reactive. Their absorbtions prevent further absorption of other species, making the catalyst inactive. These phenomena are known as catalyst poisoning.
A poison reduces the effectiveness of a catalyst. Tetraethyl lead has allways been additive to the gasoline. For environmental protection, catalytic converters have been installed in automobiles to oxidize carbon monoxide and hydrocarbons. However, lead compounds poison the ability of platinum as a catalyst. Thus, leaded gasoline should not be used for automobiles equipped with catalytic converters.
There are many types of catalyst in the market place, for example MIRATECH oxidation catalyst can also reduce carbon monoxide and hydrocarbon emissions. The most common catalytic converter uses Pt metal.
Recently, there is a concern over the reduction of sulfur in gasoline and other engine fuels for the purpose of reducing sulfur oxides emission. Technically, sulphur compounds are not catalyst poisons (i.e. they do not cause an irreversible reduction in catalyst efficiency). However, they will occupy part of the precious metal surface, thereby reducing the active conversion of exhaust emissions until the sulphur gets de-sorbed from the precious metal sites again (short-term effect).
How transition metals are chosen as catalysts?
The first period of transition metals are represented by these metals.
Sc Ti V Cr Mn Fe Co Ni Cu and Zn
Typical common features among them are the presences of d electrons, and in many of them, and their unfilled d orbitals. As a result, transition metals form compounds of variable oxidation states. Thus, these metals are electron banks that lend out electrons at appropriate time, and store them for chemical species at other times.
Tranisition metals are used in hydrogenation reactions mentioned earlier. These reaction are represented by
| |
>C=C< + 2 H -> H-C--C-H
| |
For example, the hydrogenation of unsaturated oil in the manufacture of margarine is such an application. Special catalysts such as ICT-3-25-P is made of palladium supported on the special wide-porous carbon carrier Sibunit.
Other processes catalyzed by transition metals are oxidation-reduction reactions:
NH3 + 5/4 O2 = NO + H2O
2 CO + O2 = 2 CO2
 The oxidation of CO takes place in catalytic converters, platinum is often, but not always used as a catalyst in them. The picture shown here is a dual catalytic converter showing its gas flow path.
The oxidation of CO takes place in catalytic converters, platinum is often, but not always used as a catalyst in them. The picture shown here is a dual catalytic converter showing its gas flow path.
For most transition metals except gold, the chemisorption strength follows a general sequence for gaseous reagents:
O2 > alkynes > alkenes > CO > H2 > CO2 > N2
The chemisorption strength also varies with the metals. In general, the chemisorption is the strongest for metals on the left, and it decreases for transition metals in a period as the atomic number increases:
Sc Ti V Cr Mn Fe Co Ni Cu Zn
Y Zr Nb Mo Tc Ru Rh Pd Ag Cd
La Hf Ta W Re Os Ir Pt Au Hg
The chemisorptions are too strong for Sc, Ti, V, Cr, and Mn groups and these metals are not effective catalysts.
- Fe, Ru, and Os chemisorb most gases firmly, and barely chemisorb N2.
- Chemisorb strengths for Co and Ni are weaker than those of the Fe group. Their absorptions for CO2 and H2 are very weak.
- Rh, Pd, Ir, and Pt barely chemisorb H2, but not CO2.
- Cu, Ag, barely chemisorb CO and ethylene.
These relative chemisorption strengths enable us to make some simple predictions regarding their sutability as catalysts for specific reactions. For example, a catalyst for the Haber process to produce ammonia must chemisorb nitrogen. Iron, ruthenium, or osmium may be considered.
For hydrogenation reactions, the catalyst must chemisorb H2. Metals Co, Rh, Ir, Ni, Pd, and Pt are suitable. Availability and costs are additional factors for the consideration. Nickel is actually a good choice, all considered.
These guidelines are very crude, and each case must be carefully studied. Fortunately, many catalysts are commercially available. The research and development of catalyst are left for many companies.
What are syngases and how are they prepared?
Syngas is a general term used to mean synthetic gases suitable as fuel or for the production of liquid fuel. Often, it is a mixtuure of H2 and CO, and this mixture can be converted into methanol, CH3OH. The well known catalysts are Pt and Rh, but other technology such as memberanes are also used for syngas productions.
Selection of a catalyst is important in industrial productions. For example, using rhodium or platinum as catalysts have shown to give very different distribution of products when methane or ethane were used.
CH4 (65%) + O2 (35%) ---Rh--> H2 (60%) + CO (30%) + CO2 (2%) + H2) (5%)
When platinum is used, more of the undsirable products H2O and CO2 were obtained. Swaddle has described the difference between using these two metals as catalysts (Inorganic Chemistry, page 120), but much more details is required when it comes to application. The data provided evidence to show that a slight difference in chemisorption led to very different results.
Why metal clusters will be excellent potential catalysts?
The surface area per unit weight is an important consideration when solids are used as catalysts. There are many studies related to the study of surface area of particulate metals. Various methods are developed to measure the surface areas of solid materials. One such method is the surfact area determination from gas adsorption.
Clusters are the limiting sizes of metal particles, each of which are made up only a few atoms. There is no need to rigorously define the number of atoms in a particulate to be called clusters, but a general view is that when the number of atoms at the surface of the particle is more than the nuber of atoms in the interior, the particle is a cluster. Thus, a cluster can have as few as 3 atoms, and as large as a few tens of atoms.
By the way, the term cluster have been used in other areas of study. For example, in organometallic chemistry, compounds with a few metals bonded together by metal-metal bonds are also called metal clusters. Many carbonyl compounds belong to this category. For example,
Co2(u-CO)2(CO)6, (u-CO meaning CO bridged between two metal atoms)
Mn2(CO)10
Fe3(CO)12
Co4(CO)12
Rh4(CO)12
CFe5(CO)15
Rh6(CO)16
Os6(CO)18
Metal carbonyls have been studies as homogeneous catalysts. They are mentioned here so that you will be able to appreciate their usage in other literatures.
All catalytic activities occur at the surface, because the surface atoms have tendencies for chemisorption of gas molecules. Thus, clusters will naturally be excellent potential catalysts. Thus, the study of heterogeneous catalysts may involve the study of metal cluster ion chemistry, and encapsulated silver clusters as oxidation catalysts. Clusters can be made from vapour deposition. The title of this link sounds very interesting: Metal Atom Vapor Chemistry: A Field Awaits Its Breakthrough.
Are non-stoichiometric oxides potential catalysts for redox reactions?
Due to their ability to have various oxidation state, transition metals form non-stoichiometric oxides, and they have excellent potentials for oxidation and reduction (redox) reactions, because they can both give and accept electrons.
Mn+ => M(n+1)+ + e-
M(n+1)+ + e- => Mn+
Furthermore, they resemble metals, and they catalyze hydrogenation and isomerization reactions.
A p-type metal oxide have excess positive charges in the solid, and they can adsorb oxygen to form anions such as O-, O2-, O2-, and O22- on their surfaces. Nickel oxide is such an oxide. It turns out that the adsorbed O- species is the most active,
O2 (g) + 2 Ni2+ => 2 O- (ads) + 2 Ni3+
2 O- (ads) + 2 CO (ads) => 2 CO2 + 2 e-
2 Ni3+ + 2 e- => 2 Ni2+
When an oxide gives up oxygen, electrons were left behind and the negative charge in it makes it a n-type oxide. Zinc oxide is such an n-type oxide, and the reaction mechanism may be represented as follows:
CO (g) + 2 O2- (lattice) => CO32- (lattice) + 2 e-
0.5 O2 + 2 e- => O2-
CO32- (lattice) = CO2 + O2- (lattice)
The overall reaction is ---
CO + 0.5 O2 => CO2
In these primary steps, the oxygen is consumed via adsorption on the solid.
A sulfide, such as MoS2, can loose sulfur atoms to become a n-type solid, Mo1+xS2 or gain a sulfur atom to become a p-type solid Mo1-xS2, depending on the vapour pressure of S2 gas surrounding the solid. By doping MoS2 with oxide can also make it a p-type solid for a catalyst.
One of the useful applications of MoS2 as a catalyst is to reduce sulfur in gasoline. For example, the cyclic thiophene C4H4S can be converted to a hydrocarbon by using a p-type MoS2,
C4H4S + 4 H2 == p-type MoS2 == > C4H10 + H2S
This is accomplished by a typical commercial hydrodesulfurization catalyst, which may contain 14% MoO3, and 3% CoO on alumina support.
What type of catalysts can be made of stoichiometric oxides?
It has been well known that metal oxides dissolve in water to form basic solutions whereas non-metalic oxides dissolve in water to give acidic solutions. Some metal oxides such as Al2O3, Fe2O3, Cr2O3 etc dissolve in strong acid and bases. Thus, we can divide oxides into acidic and basic oxides for catalytic activities.
Acidic Oxides
Acidic oxides such as Al2O3 and SiO2 catalyze dehydration reactions such as
R-CH2CH2OH (g) == (Al2O3, 600 K) ==> R-CH=CH2
If we consider the oxide a Lewis acid, it adsorbs the OH group, facilitating the reaction in the following steps.
R-CH2CH2OH (g) => R-CH2-CH2+ + OH- (adsorbed)
R-CH2-CH2+ => R-CH+-CH3
R-CH+-CH3 + OH- (adsorbed) => R-CH=CH2 + H2O
Zeolites, which are alumniosilicates, function as acidic catalysts. They also catalyze isomerization, cracking, alkylation and other organic reactions.
Basic Oxides
Basic oxides such as MgO and ZrO favor reactions involving anionic species. When a proton, H+, is adsorbed onto the surface close to an O2- ion in the metal oxide, an OH- group is formed, leaving the organic molecule a negative charge.
CH3-CH2-CN + MO (solid) => -CH2-CH2CN + M-OH+ (solid)
=> CH2=CH-CN + MOH2 (solid)
= + oxygen => CH2=CH-CN + MO (solid) + H2O (product)
The over all reaction is a selective oxidation
CH3-CH2-CN + MO (solid) + 0.5 O2 => CH2=CH-CN + MO (solid) + 0.5 H2O
The oxidation eliminated two hydrogen atoms per molecule in the process, and the proposed mechanism suggests a two step elimination process.
Mixtures of basic oxides have been used as catalysts in the oxidative coupling of methane. In some cases, special reactors and catalysts are designed for this type of application. TAP Reactor is one such an application. In this case, zeolites were used.
What are photocatalytic reactions?
Reactions caused by photons, bundles of radiation energy, are called photolysis. Photocatalyic reactions imply photolysis in the presence of a catalyst. In most cases, however, the catalysts are semiconductors and the reactions are semiconductor assisted photolysis reactions. In this aspect, the photocatalyst has a slightly different function than those in thermal chemical process.
The simulation below shows that when the yellow beam strikes the semiconductor TiO2, an electrons are excited from the valance band into the conduction band. This band gap is 3.2 V. The excited electron then promote the production of H2. The holes take electrons from OH- groups converting them to active OH radicals. The radicals break up forming O2 or react with CHCl3 converting it into the harmless CO2, H+, and Cl-. This simulation of photocatalysts is prepared by a Japanese group, and it illustrates the concept rather well. In reality, the process is rather complicated.
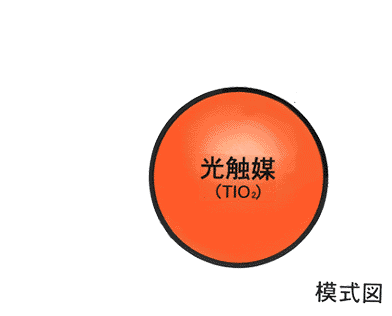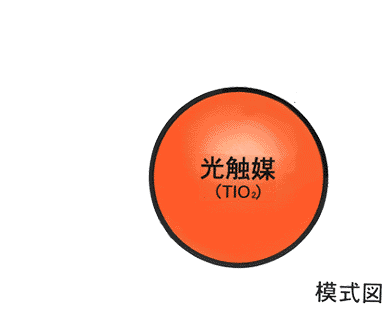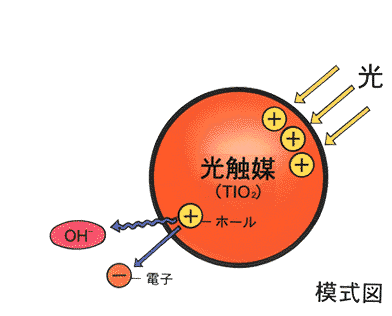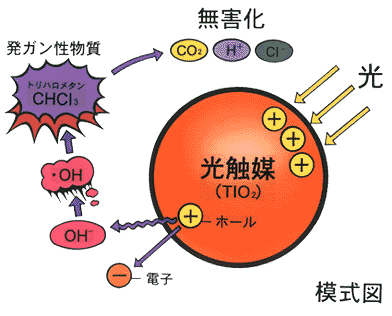
In the photodecomposition of water, the excited electrons react with hydrogen ions (protons)
2 H+ + 2 e- = H2
2 OH- + 2 e+ (hole) = H2O + 0.5 O2
Thus, the products, H2 and O2, are potential fuels for the supply of energy, especially for fuel cells.
As another example, fluoroboric acid is used in electroplating and metal finishing. To treat wastewater from these industries requires the removal of fluoroboric acid. Existing methods of adsorption, coagulation, precipitation methods do not work. Thus, photocatalytic decomposition of fluoroboric acid has been studied, and it showed that TiO2 being rather effective when it doped with Cr and Fe oxides. The above link showed that doping of Cr or Fe drastically enhanced the activity. Moreover, 0.5 wt% Cr/TiO2 and 1.0 wt% Fe/TiO2 showed maximum activities of 61 % and 41 %, respectively.
Recently, a news article has an attractive claim on Indoor Air Cleaner. Judge it yourself to see if it is something worth investigating.
The energy gap of TiO2 is 3.2 V. What is the frequency of the photons that has just eneough energy to excite the electrons from the covalent band of TiO2 into the conduction band?
Solution
The energy to excite an electron up 3.2 V is 3.2 eV.
1.6022e-19 J 1
3.2 eV ------------- ------------- = 7.74e14 Hz
1 eV 6.626e-34 J s
DISCUSSION
The wavelength of these photons are
3e8 m/s
-------- = 387e9 m (or 387 nm)
7.74 /s
These photons are at the just within visible limit of 350 - 700 nm.
A mole of photons is called an einstein. How much energy in J is an einstein of the photons described in Example 1.
Solution
The energy is
1.6022e-19 J 6.022e23
3.2 eV -------------- ------------- = 308000 J = 308 kJ
1 eV 1 photon
DISCUSSION
When we discuss Gibbs energy, we have learned that the enthalpy of formation for H2O is - 285.83 kJ. This means that we need a minimum of 286 kJ to decompose water. Thus, 1 einstein of photons has more energy to decompose a mole of water than the minimum. However, an overpotential is required to decompose water. The titanium oxide is mixed with platinum metal and ruthenium oxide to facilitate the formation of bubbles in these process. (See Inorganic Chemistry by Swaddle(page 125).
Questions
- What is the difference between phsysisorption and chemisorption?
Solutions
- Increase rates of reaching chemical equilibrium. Selectively increase certain products. Decompose undesirable products... and more.
- When chemical bonds are formed or broken, it's chemisorption.
- False. A strongly adsorbed species poisons a catalyst.
- oxygen
- Fe
- 0.25
Contributors and Attributions
Chung (Peter) Chieh (Professor Emeritus, Chemistry @ University of Waterloo)

