Colinergic Drugs II - Anticholinesterase Agents & Acetylcholine Antagonists
- Page ID
- 89061
Anticholinesterase Agents
Acetylcholine is inactivated by the enzyme acetylcholinesterase (enlarged), which is located at cholinergic synapses and breaks down the acetylcholine molecule into choline and acetate. Three particularly well-known drugs, neostigmine, physostigmine, and diisopropyl fluorophosphate, inactivate acetylcholinesterase so that it cannot hydrolyze the acetylcholine released at the nerve ending. As a result, acetylcholine increases in quantity with successive nerve impulses so that large amounts of acetylcholine can accumulate and repetitively stimulate receptors. In view of the widespread distribution of cholinergic neurons, it is not surprising that the anticholinesterase agents as a group have received more extensive application as toxic agents, in the form of agricultural insecticides and potential chemical-warfare "nerve gases," than as therapeutic agents. Nevertheless, several members of this class of compounds are clinically useful.
The active center of acetylcholinesterase consists of a negative subsite, which attracts the quaternary group of choline through both coulombic and hydrophobic forces, and an esteratic subsite, where nucleophilic attack occurs on the acyl carbon of the substrate. The catalytic mechanism resembles that of other serine esterases, where a serine hydroxyl group is rendered highly nucleophilic through a charge-relay system involving the close apposition of an imidazole group and , presumably, a carboxyl group on the enzyme. During enzymatic attack on the ester, a tetrahedral intermediate between enzyme and ester is formed that collapses to an acetyl enzyme conjugate with the concomitant release of choline. The acetyl enzyme is labile to hydrolysis, which results in the formation of acetate and active enzyme. Acetylcholinesterase is one of the most efficient enzymes known and has the capacity to hydrolyze \(3 \times 10^5\) acetylcholine molecules per molecule of enzyme per minute: this is equivalent to a turnover time of 150 microseconds.
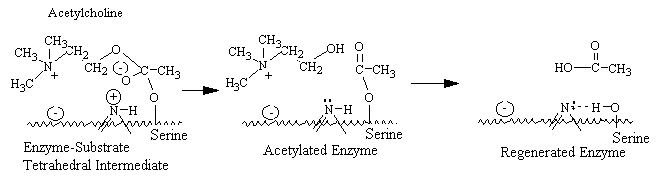
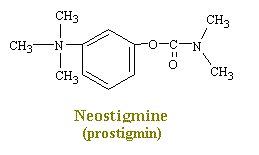
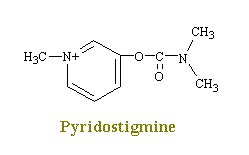
Drugs such as neostigmine and pydrostigmine that have a carbamyl ester linkage are hydrolyzed by acetylcholinesterase, but much more slowly than acetylcholine because they from more stable intermediates. Therefore, these drugs inactivate acetylcholinesterase for up to several hours, after which they are displaced so that acetylcholinesterase. These drugs are used for their effects on skeletal muscle and the eye (pupillary constriction and decreased intraocular pressure) and for the treatment of atropine poisoning. On the other hand, diisopropyl fluorophosphate, which has military potential as a powerful nerve gas poison, inactivates acetylcholinesterase for weeks, which makes this a particularly lethal poison.
Generally the pharmacological properties of anticholinesterase agents can be predicted merely by knowing those loci where acetylcholine is released physiologically by nerve impulses. and the responses of the corresponding effector organs to acetylcholine. While this is true of the main, the diverse locations of cholinergic synapses increase the complexity of the response. Potentially, the antcholiesterase agents can produce all of the following effects: 1) stimulation of muscarinic receptor responses at autonomic effector organs; 2) stimulation, followed by paralysis, of all autonomic ganglia and skeletal muscle; and 3) stimulation, with occasional subsequent depression, of cholinergic receptor sites in the CNS. However, with smaller doses, particularly those employed therapeutically, several modifying factors are significant.
Neostigmine and Pydrostigmine
Neostigmine and pydrostigmine are among the principal anticholinesterases. These drugs have only a few clinical uses, mainly in augmenting gastric and intestinal contractions (in treatment of obstructions of the digestive tract), in generally augmenting muscular contractions (in the treatment of myasthenia gravis), and in constricting the eye pupils (in the treatment of glaucoma). Other anticholinesterases in larger doses, however, are widely used as toxins that achieve their effects by causing a continual stimulation of the parasympathetic nervous system. Parathion and malathion are thus highly effective agricultural insecticides, while tabun and serin are nerve gases used in chemical warfare to induce nausea, vomiting, convulsions, and death in humans. This action produces a decrease in the rate of destruction of acetylcholine in the synaptic cleft and hence an increase in the amount of transmitter available to interact with the receptors.
Serin Nerve Gas
Acetylcholine Antagonists
Atropine, a naturally occurring alkaloid of "Atropa belladonna", the deadly nightshade, inhibits the actions of acetylcholine on autonomic effectors innervated by postganglionic cholinergic nerves as well as on smooth muscles that lack cholinergic innervation. Since atropine antagonizes the muscarinic actions of acetylcholine, it is known as an antimuscarinic agent. Evidence shows that atropine and related compounds compete with muscarinic agonists for identical binding sites on muscarinic receptors.
In general, antimuscarinic agents have only a moderate effect on the actions of acetylcholine at nicotinic receptor sites. Thus, at autonomic ganglia, where transmission primarily involves an action of acetylcholine on nicotinic receptors, atropine produces only partial block. At the neuomuscular junction, where the receptors are exclusively nicotinic, extremely high doses of atropine or related drugs are required to cause any degree of blockade. In the central nervous system, cholinergic transmission appears to be predominantly nicotinic in the spinal chord and both nicotinic and muscarinic in the brain. Many of the CNS effects of atropine-like drugs are probably attributable to their central antimuscarinic actions.
Atropine
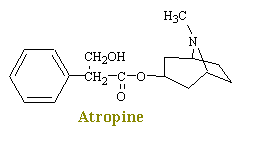
When used as premedication for anaesthesia, atropine decreases bronchial and salivary secretions, blocks the bradycardia associated with some drugs used in anaesthesia such as halothane, suxamethonium and neostigmine, and also helps prevent bradycardia from excessive vagal stimulation.
There is usually an increase in heart rate and sometimes a tachycardia as well as inhibition of secretions (causing a dry mouth) and relaxation of smooth muscle in the gut, urinary tract and biliary tree. Since atropine crosses the blood brain barrier CNS effects in the elderly may include amnesia, confusion and excitation. Pupillary dilatation and paralysis of accommodation occur, with an increase in intraocular pressure especially in patients with glaucoma. Occasionally small intravenous doses may be accompanied by slowing of the heart rate due to a central effect - this resolves with an extra increment of intravenous atropine Being a sympathetic cholinergic blocking agent, signs of parasympathetic block may occur such as dryness of the mouth, blurred vision, increased intraocular tension and urinary retention.
Sarin is a nerve agent in the organophosphate family. It is dispersed in a droplet or mist form. Upon inhalation, for instance, the symptoms (in order of occurrence) include: runny nose, bronchial secretions, tightness in the chest, dimming of vision, pin-point pupils, drooling, excessive perspiration, nausea, vomiting, involuntary defecationand urination, muscle tremors, convulsions, coma, and death. Primary treatment for nerve agents is atropine sulfate. It is commonly carried in auto-injectors by military personnel in dosages of 1-2 mgs. However, in many cases, massive doses may be necessary to reverse the effects of the anticholinesterase agents. Frequently, 20-40 mgs. of atropine may be necessary.

Picture of Auto-Injector
Curare
In 1799 the famous Prussian explorer and scientist Baron Von Humboldt discovered a potent drug called curare. On an expedition into the jungles of Venezuela, he watched an Indian hunter bring down a large animal with a single shot from his bow and arrow. The arrow had been poisoned with curare, a potion with two curious properties, derived from the jungle plants. Curare injected into the bloodstream, as it was when hunting animals, was deadly. It immobilized the body, attacked the vital organs, and caused death almost instantaneously. Humboldt discovered the second property of curare in a more dramatic fashion. He became sick, and a native witch doctor forced Humboldt to drink some curare that had been diluted with water. Terrified that he was going to die, Humboldt was surprised to find that after drinking the curare, he felt significantly better. Curare, when it was diluted and taken orally, he discovered, could have a positive medicinal value without causing any damage to vital organs.
Curare is a generic term for various South American arrow poisons. The main active ingredient in curare is d-tubocurarine, which has the chemical structure shown below.
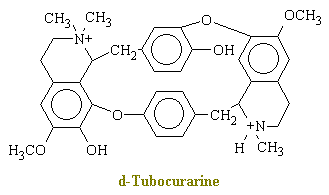
In brief, d-tubocurarine is an antagonist of the cholinergic receptor sites at the post junctional membrane and thereby blocks competitively the transmitter action of acetylcholine. When the drug is applied directly to the end-plate of a single isolated muscle fiber under microscopic control, the muscle cell becomes insensitive to motor-nerve impulses and to direct applied acetylcholine; however, the end -plate region and the remainder of the muscle fiber membrane retain their normal sensitivity to the application of potassium ions, and the muscle fiber still responds to direct electrical stimulation. Because acetylcholine release into the neuromuscular junction muscle is what initiates contraction, curare causes muscle relaxation and paralysis.
There are several clinical application for neuromuscular blockage. The most important by far is the induction of muscle relaxation during anesthesia for effective surgery. Without such drugs deeper anesthesia, requiring more anesthetic, would be needed to achieve the same degree of muscle relaxation: tracheal intubation would also be impossible because of strong reflex response to tube insertion. It is the decreased need for anesthetics, however, that represents increased surgical safety.
Neuromuscular blockers also find limited utility in convulsive situations such as those precipitated by tetanus infections and to minimize injury to patients undergoing electroconvulsive therapy. Manipulation of fractured or dislocated bones may also be aided by such drugs.
Botulus Toxin
Botulus toxin* is a bacterial poison that prevents the release of acetylcholine by all types of cholinergic nerve terminals. Apparently, the toxin blocks release of vesicular acetylcholine at the preterminal portion of the axon, but why this is confined to cholinergic fibers is not known.

