2.5: Rules for Resonance Forms
- Page ID
- 239079
Objectives
After completing this section, you should be able to
- use the concept of resonance to explain structural features of molecules and ions.
- understand the relationship between resonance and relative stability of molecules and ions.
Rules for Drawing and Working with Resonance Contributors
Recognizing, drawing, and evaluating the relative stability of resonance contributors is essential to understanding organic reaction mechanisms. When learning to draw and interpret resonance structures, there are a few basic guidelines to help. .
1) There is ONLY ONE REAL STRUCTURE for each molecule or ion. This real structure (the resonance hybrid) takes its character from the average of all the individual resonance contributors. When looking at a resonance contributors, we are seeing the exact same molecule or ion depicted in different ways. Resonance hybrids are really a single, unchanging structure.
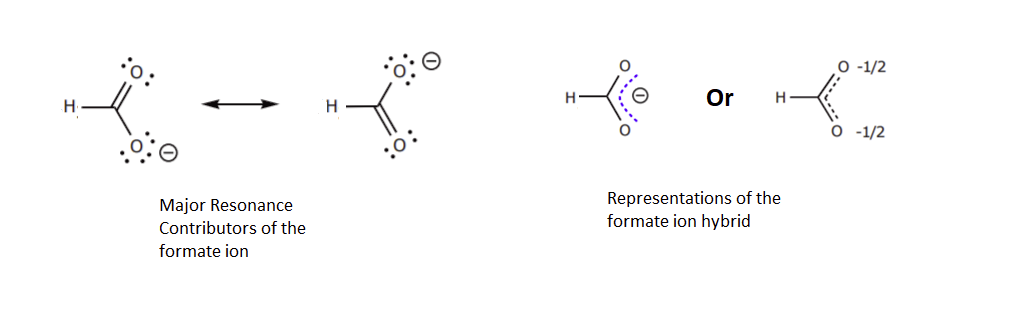
This picture is from an outside source
2) The resonance hybrid is more stable than any individual resonance structures. Often, resonance structures represent the movement of a charge between two or more atoms. The charge is spread out amongst these atoms and therefore more stabilized. When looking at the picture above the resonance contributors represent the negative charge as being on one oxygen or the other. The resonance hybrid shows the negative charge being shared equally between two oxygens. In the resonance hybrid, the negative charge is spread out over a larger part of the molecule and is therefore more stable.
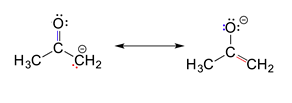
3) Resonance contributors do not have to be equivalent. Because of this, resonance structures do necessarily contribute equally to the resonance hybrid. The two resonance structures shown below are not equivalent because one show the negative charge on an oxygen while the other shows it on a carbon. Later, we will show that the contributor with the negative charge on the oxygen is the more stable of the two. Also, this means that the resonance hybrid will not be an exact mixture of the two structures.
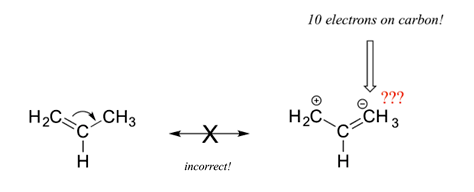
4) All resonance contributors must be correct Lewis structures. Each atom should have a complete valence shell and be shown with correct formal charges. A carbocation (carbon with only 6 valence electrons) is the only allowed exception to the valence shell rules. The structure below is an invalid resonance structure even though it only shows the movement of a pi bond. The resulting structure contains a carbon with ten electrons, which violates the octet rule, making it invalid.
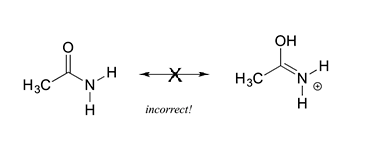
5) All resonance contributors must have the same molecular formula, the same number of electrons, and same net charge. The molecules in the figure below are not resonance structures of the same molecule because then have different molecular formulas (C2H5NO Vs. C2H6NO). Also, the two structures have different net charges (neutral Vs. positive).
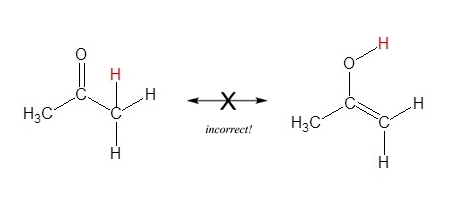
6) Resonance contributors only differ by the positions of pi bond and lone pair electrons. Sigma bonds are never broken or made, because of this atoms must maintain their same position. The molecules in the figure below are not resonance structures of the same molecule even though they have the same molecular formula (C3H6O). These molecules are considered structural isomers because their difference involves the breaking of a sigma bond and moving a hydrogen atom.
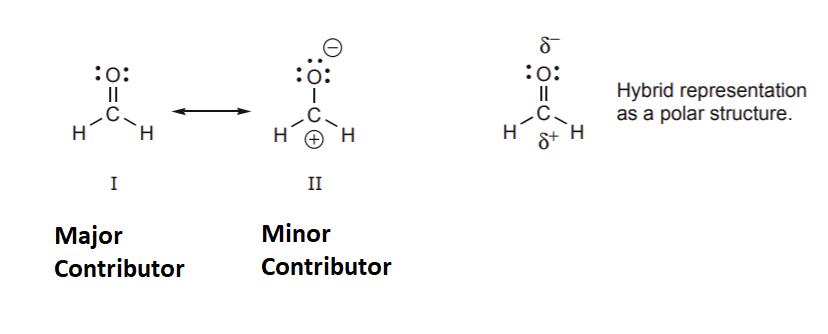
Major and Minor Resonance Contributors
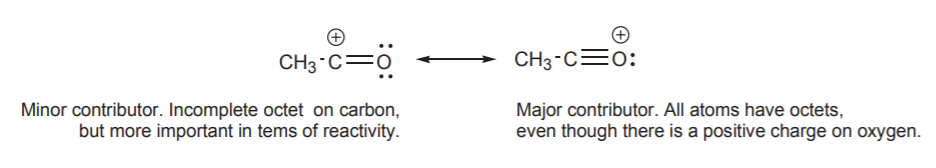
As previously state the true structure of a resonance hybrid is the combination of all the possible resonance structures. If the resonance structures are equal in stability they the contribute equally to the structure of the hybrid. However, if the resonance structures have different stabilities they contribute to the hybrid's structure in proportions related to their relative stabilities. It can be said the the resonance hybrid's structure resembles the most stable resonance structure. Because of this it is important to be able to compare the stabilities of resonance structures. In the example below, structure II is much less important in terms of its contribution to the hybrid because it contains the violated octet of a carbocation. The relative stabilities of the two structures are so vastly different that molecules which contain a C=O bond are almost exclusively written in a form like structure I. However, as will learn in chapter 19, the positively charged carbon created by structure II will explain how the C=O bond will react with electron rich species.
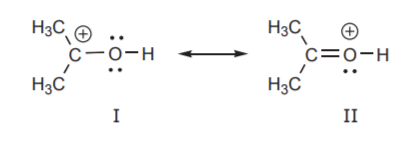
Rules for Estimating Stability of Resonance Structures
1. The resonance structures in which all atoms have complete valence shells is more stable. This means most atoms have a full octet. In the example below structure I has a carbon atom with a positive charge and therefore an incomplete octet. Based on this criterion,structure I is less stable and is a more minor contributor to the resonance hybrid than structure II.
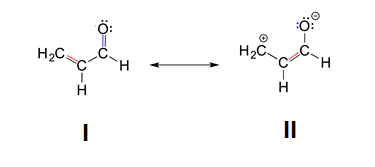
2. The structures with the least number of formal charges is more stable. Based on this, structure II is less stable because is has two atoms with formal charges while structure I has none. Structure I would be the major resonance contributor.
3. The structures with a negative charge on the more electronegative atom will be more stable. The difference between the two resonance structures is the placement of a negative charge. Structure II is the more stable and the major resonance contributor, because it places the negative charge on the more electronegative oxygen.
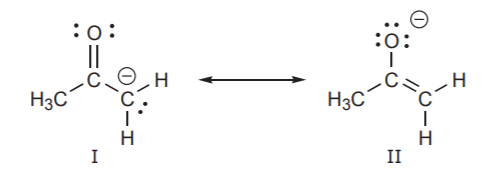
4. The structures with a positive charges on the least electronegative atom (most electropositive) is more stable.
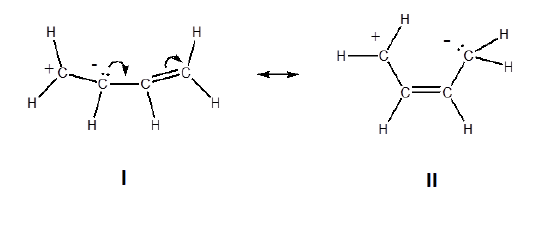
5. The structures with the least separation of formal charges is more stable. The only difference between the two structures below are the relative positions of the positive and negative charges. In structure I the charges are closer together making it more stable.
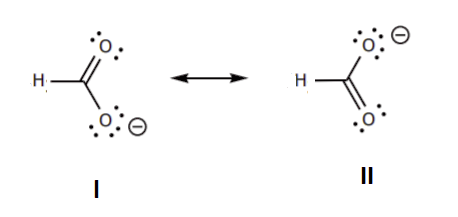
6. Resonance forms that are equivalent have no difference in stability. When looking at the two structures below no difference can be made using the rules listed above. This means the two structures are equivalent in stability and would make equal structural contributions to the resonance hybrid.
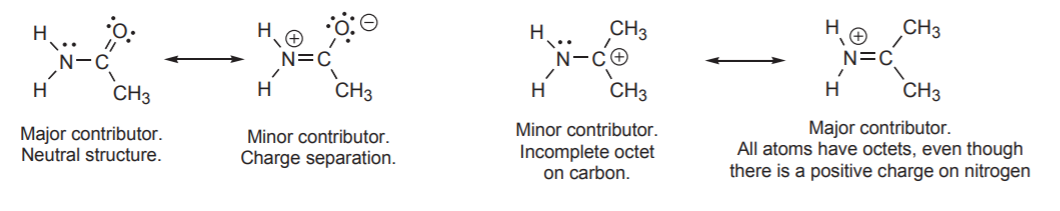
Example
Exercises
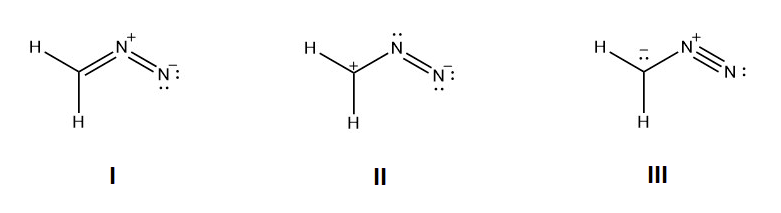
1) For the following resonance structures please rank them in order of stability. Indicate which would be the major contributor to the resonance hybrid.
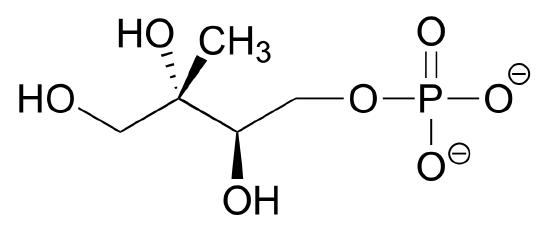
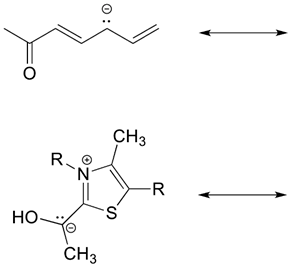
2) Draw four additional resonance contributors for the molecule below. Label each one as major or minor (the structure below is of a major contributor).
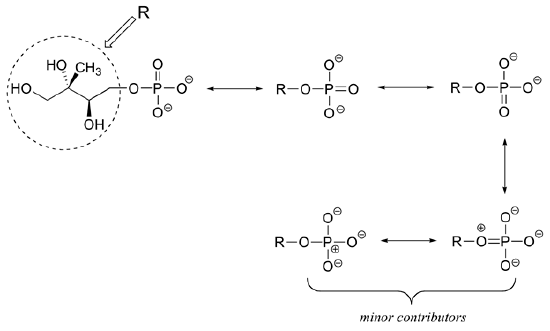
3) Draw three resonance contributors of methyl acetate (an ester with the structure CH3COOCH3), and order them according to their relative importance to the bonding picture of the molecule. Explain your reasoning.
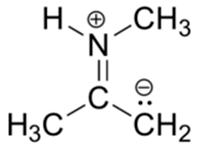
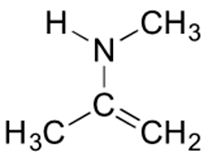
4) Below is a minor resonance contributor of a species known as an ‘enamine’, which we will study more in Section 23.12. Draw the major resonance contributor for the enamine, and explain why your contributor is the major one.
Solutions
1) Structure I would be the most stable because all the non-hydrogen atoms have a full octet and the negative charge is on the more electronegative nitrogen. Structure III would be the next in stability because all of the non-hydrogen atoms have full octets. Structrure II would be the least stable because it has the violated octet of a carbocation.
2)
3)
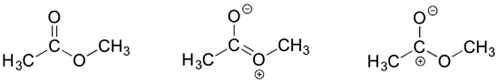
The contributor on the left is the most stable: there are no formal charges.
The contributor on the right is least stable: there are formal charges, and a carbon has an incomplete octet.
The contributor in the middle is intermediate stability: there are formal charges, but all atoms have a complete octet.
4) This contributor is major because there are no formal charges.
5)
Contributors and Attributions
Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)
Prof. Steven Farmer (Sonoma State University)
William Reusch, Professor Emeritus (Michigan State U.), Virtual Textbook of Organic Chemistry
Organic Chemistry With a Biological Emphasis by Tim Soderberg (University of Minnesota, Morris)

