Example Emphasized Terms
- Page ID
- 412054
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)After completing this section, you should be able to
- calculate the maximum number of stereoisomers possible for a compound containing a specified number of chiral carbon atoms.
- draw wedge-and-broken-line structures for all possible stereoisomers of a compound containing two chiral carbon atoms, with or without the aid of molecular models.
- assign R,S configurations to wedge-and-broken-line structures containing two chiral carbon atoms, with or without the aid of molecular models.
- determine, with or without the aid of molecular models, whether two wedge-and-broken-line structures containing two chiral carbon atoms are identical, represent a pair of enantiomers, or represent a pair of diastereomers.
- draw the wedge-and-broken-line structure of a specific stereoisomer of a compound containing two chiral carbon atoms, given its IUPAC name and R,S configuration.
Make certain that you can define, and use in context, the key term below.
- diastereomer
Diastereomers are two molecules which are stereoisomers (same molecular formula, same connectivity, different arrangement of atoms in space) but are not enantiomers. Unlike enatiomers which are mirror images of each other and non-sumperimposable, diastereomers are not mirror images of each other and non-superimposable. Diastereomers can have different physical properties and reactivity. They have different melting points and boiling points and different densities. In order for diastereomer stereoisomers to occur, a compound must have two or more stereocenters.
Introduction
So far, we have been analyzing compounds with a single chiral center. Next, we turn our attention to those which have multiple chiral centers. We'll start with some stereoisomeric four-carbon sugars with two chiral centers.
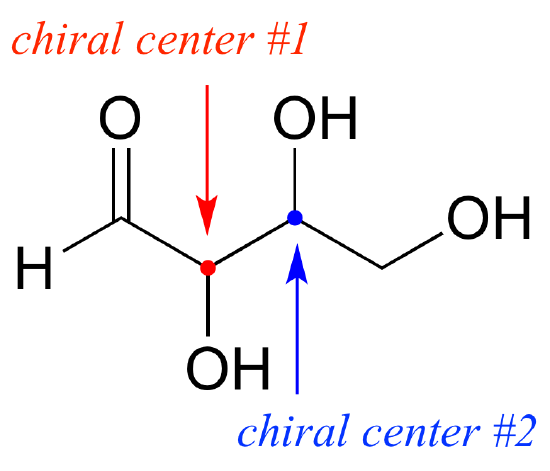
We will start with a common four-carbon sugar called D-erythrose.
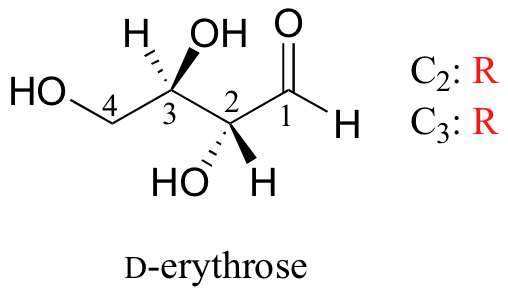
A note on sugar nomenclature: biochemists use a special system to refer to the stereochemistry of sugar molecules, employing names of historical origin in addition to the designators 'D' and 'L'. You will learn about this system if you take a biochemistry class. We will use the D/L designations here to refer to different sugars, but we won't worry about learning the system.
As you can see, D-erythrose is a chiral molecule: C2 and C3 are stereocenters, both of which have the R configuration. In addition, you should make a model to convince yourself that it is impossible to find a plane of symmetry through the molecule, regardless of the conformation. Does D-erythrose have an enantiomer? Of course it does – if it is a chiral molecule, it must. The enantiomer of erythrose is its mirror image, and is named L-erythrose (once again, you should use models to convince yourself that these mirror images of erythrose are not superimposable).
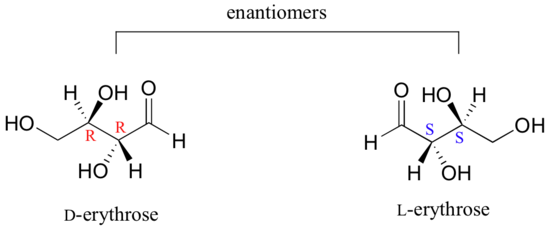
Notice that both chiral centers in L-erythrose both have the S configuration. To avoid confusion, we will simply refer to the different stereoisomers by capital letters.
Now let's consider all the possible stereoisomers
Look first at compound A below. Both chiral centers in have the R configuration (you should confirm this for yourself!). The mirror image of Compound A is compound B, which has the S configuration at both chiral centers. If we were to pick up compound A, flip it over and put it next to compound B, we would see that they are not superimposable (again, confirm this for yourself with your models!). A and B are nonsuperimposable mirror images: in other words, enantiomers.
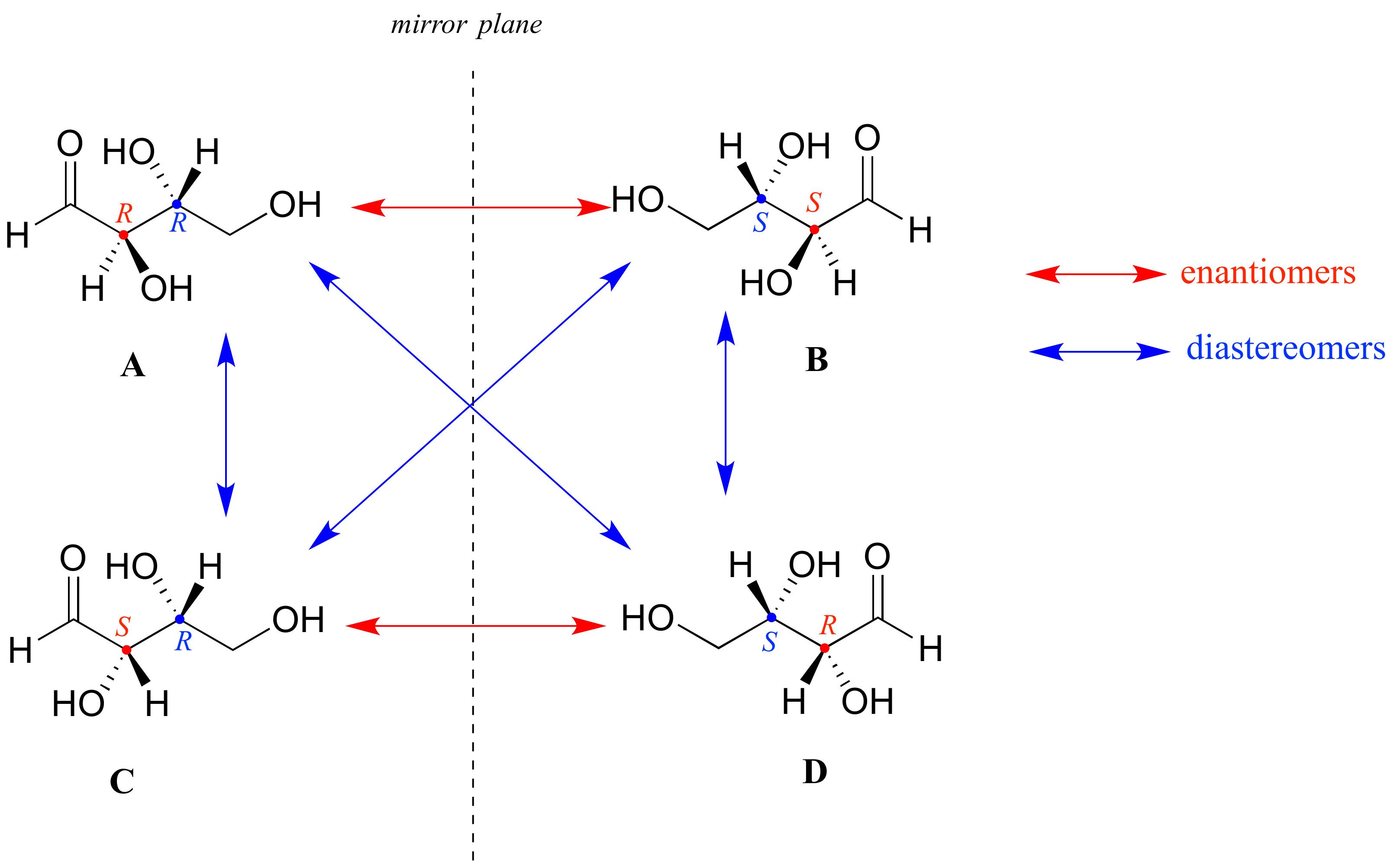
Now, look at compound C, in which the configuration is S at chiral center 1 and R at chiral center 2. Compounds A and C are stereoisomers: they have the same molecular formula and the same bond connectivity, but a different arrangement of atoms in space (recall that this is the definition of the term 'stereoisomer). However, they are not mirror images of each other (confirm this with your models!), and so they are not enantiomers. By definition, they are diastereomers of each other.
Notice that compounds C and B also have a diastereomeric relationship, by the same definition.
So, compounds A and B are a pair of enantiomers, and compound C is a diastereomer of both of them. Does compound C have its own enantiomer? Compound D is the mirror image of compound C, and the two are not superimposable. Therefore, C and D are a pair of enantiomers. Compound D is also a diastereomer of compounds A and B.
This can also seem very confusing at first, but there some simple shortcuts to analyzing stereoisomers:
Stereoisomer Shortcuts
If all of the chiral centers are of opposite R/S configuration between two stereoisomers, they are enantiomers.
If at least one, but not all of the chiral centers are opposite between two stereoisomers, they are diastereomers.
These shortcuts to not take into account the possibility of additional stereoisomers due to alkene groups: we will come to that later
Here's another way of looking at the four stereoisomers, where one chiral center is associated with red and the other blue. Pairs of enantiomers are stacked together.

We know, using the shortcut above, that the enantiomer of RR must be SS - both chiral centers are different. We also know that RS and SR are diastereomers of RR, because in each case one - but not both - chiral centers are different.
Determining the Maximun Number of Stereoisomers for a Compound
In general, a structure with n stereocenters will have a maximum of 2n different stereoisomers. (We are not considering, for the time being, the stereochemistry of double bonds – that will come later). For example, let's consider the glucose molecule in its open-chain form (recall that many sugar molecules can exist in either an open-chain or a cyclic form). There are two enantiomers of glucose, called D-glucose and L-glucose. The D-enantiomer is the common sugar that our bodies use for energy. It has n = 4 stereocenters, so therefore there are 2n = 24 = 16 possible stereoisomers (including D-glucose itself).
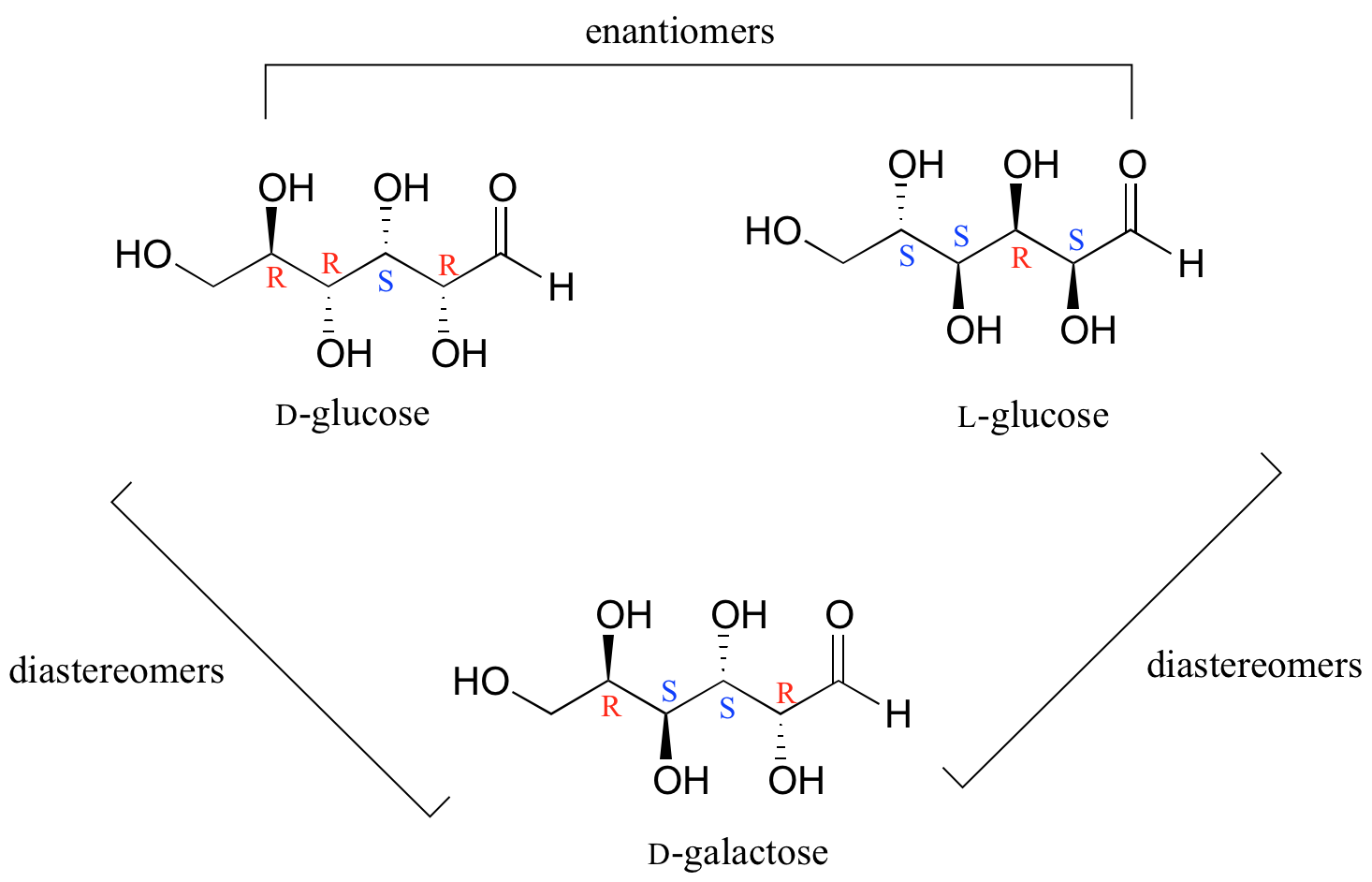
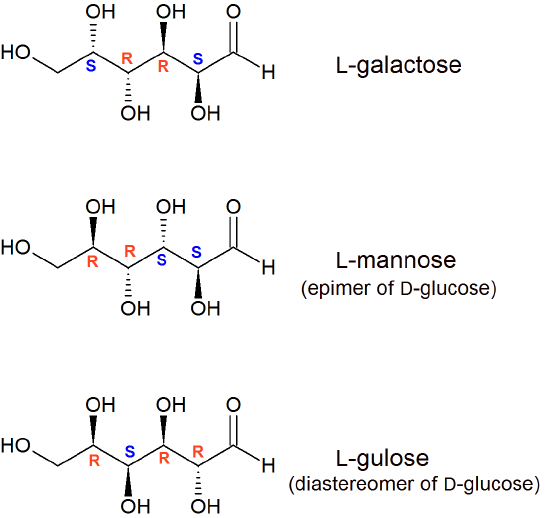
In L-glucose, all of the stereocenters are inverted relative to D-glucose. That leaves 14 diastereomers of D-glucose: these are molecules in which at least one, but not all, of the stereocenters are inverted relative to D-glucose. One of these 14 diastereomers, a sugar called D-galactose, is shown above: in D-galactose, one of four stereocenters is inverted relative to D-glucose. Diastereomers which differ in only one stereocenter (out of two or more) are called epimers. D-glucose and D-galactose can therefore be refered to as epimers as well as diastereomers.
- Draw the structure of L-galactose, the enantiomer of D-galactose.
- Draw the structure of two more diastereomers of D-glucose. One should be an epimer.
- Answer
Erythronolide B, a precursor to the 'macrocyclic' antibiotic erythromycin, has 10 stereocenters. It’s enantiomer is that molecule in which all 10 stereocenters are inverted.
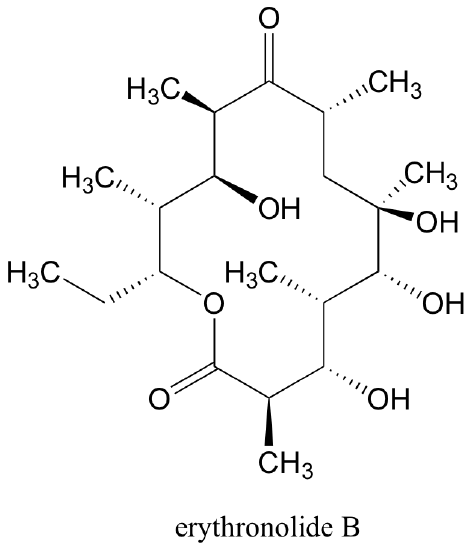
In total, there are 210 = 1024 stereoisomers in the erythronolide B family: 1022 of these are diastereomers of the structure above, one is the enantiomer of the structure above, and the last is the structure above.
We know that enantiomers have identical physical properties and equal but opposite degrees of specific rotation. Diastereomers, in theory at least, have different physical properties – we stipulate ‘in theory’ because sometimes the physical properties of two or more diastereomers are so similar that it is very difficult to separate them. In addition, the specific rotations of diastereomers are unrelated – they could be the same sign or opposite signs, and similar in magnitude or very dissimilar.
Determine the number of stereoisomers a molecule can have with…
- 3 chiral centers
- 1 chiral center
- 6 chiral centers
- Answer
-
Since a molecule with n chiral centers can have 2n stereoisomers…
- 23 = 8 possible stereoisomers
- 21 = 2 possible stereoisomers
- 26 = 64 possible stereoisomers
What is the relationship between enantiomers?
- Answer
-
They are mirror images of each other and when 2 or more chiral centers are present, every stereocenter is the opposite in its enantiomer.
How does the stereochemistry in diastereomers differ from each other?
- Answer
-
In diastereomers, one or more of the chiral centers is the opposite but they all can’t be the opposite or else they’d be enantiomers.
What are epimers?
- Answer
-
Epimers are when only one chiral center is the opposite (in molecules with 2 or more chiral centers) in its diastereomer.
Draw the structure of (2R,3R) 2-fluoro-3-methylhexane.
- Answer
-
Draw both diastereomers of (2R,3R) 2-fluoro-3-methylhexane.
- Answer
-
Draw the enantiomer of (2R,3R) 2-fluoro-3-methylhexane.
- Answer
-
Draw the structure of L-galactose, the enantiomer of D-galactose.
- Answer
-
Draw a diastereomer of D-galactose that is an epimer.
- Answer
-
You can draw an epimer by drawing D-galactose with 1 (and only 1) of its chiral centers reversed. Here’s an example when you switch only the first chiral center (in red). (There are 3 other epimers that could be drawn as long as you only swap a single chiral center in the diastereomer that you use.)
Identify if the following diastereomer of galactose is an epimer of D- galactose or L- galactose.
- Answer
-
Since the diastereomer above only varies from L-galactose by 1 chiral center, the above is an epimer in relationship to L-galactose. Since it varies from D-galactose by 3 chiral centers, it is not an epimer but a diastereomer. Since not all of the chiral centers are swapped, it is not an enantiomer!
For the compound shown below, label each chiral center as R or S.
- Answer
-
How many stereoisomers are possible for the compound in part a)?
- Answer
-
Since there are 3 chiral centers, 23 = 8 possible stereoisomers.
Consider the stereoisomers below.
- Which is/are an enantiomer of i?
- Which is/are a diastereomer of ii?
- Which is/are an epimer of i?
- Answer
-
- iv is an enantiomer of i since both chiral centers are switched and they are non superimposable mirror images.
- i & iv are diastereomers of ii since they are stereoisomers that are not mirror images.
- ii and iii are epimers of i since they are diastereomers with only 1 chiral center switched and the other one the same.
Consider the 8 stereoisomers below.
- Which is/are an enantiomer of i?
- Which is/are a diastereomer of i?
- Which is/are an epimer of i?
- Answer
-
- v is an enantiomer since all three chiral centers are switched and they are non superimposable mirror images.
- ii, iii, iv, vi, vii & viii are diastereomers of i since they are stereoisomers that are not mirror images.
- ii,iii & viii are epimers of i since they are diastereomers with only 1 chiral center switched and the other chiral centers the same.

