E5: Acid Dissociation Constants of Organics
- Page ID
- 6647
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)The following table provides pKa and Ka values for selected weak acids. All values are from Martell, A. E.; Smith, R. M. Critical Stability Constants, Vols. 1–4. Plenum Press: New York, 1976. Unless otherwise stated, values are for 25 oC and for zero ionic strength. Those values in brackets are considered less reliable.
Weak acids are arranged alphabetically by the names of the neutral compounds from which they are derived. In some cases—such as acetic acid—the compound is the weak acid. In other cases—such as for the ammonium ion—the neutral compound is the conjugate base. Chemical formulas or structural formulas are shown for the fully protonated weak acid. Successive acid dissociation constants are provided for polyprotic weak acids; where there is ambiguity, the specific acidic proton is identified.
To find the Kb value for a conjugate weak base, recall that
\[K_\text{a} \times K_\text{b} = K_\text{w} \nonumber\]
for a conjugate weak acid, HA, and its conjugate weak base, A–.
| compound | conjugate acid | pKa | Ka |
|---|---|---|---|
| acetic acid | \(\ce{CH3COOH}\) | 4.757 | \(1.75 \times 10^{-5}\) |
| adipic acid |  |
4.42 5.42 |
\(3.8 \times 10^{-5}\) \(3.8 \times 10^{-6}\) |
| alanine | 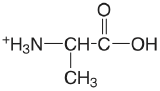 |
2.348 (\(\ce{COOH}\)) 9.867 (\(\ce{NH3}\)) |
\(4.49 \times 10^{-3}\) \(1.36 \times 10^{-10}\) |
| aminobenzene | 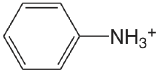 |
4.601 | \(2.51 \times 10^{-5}\) |
| 4-aminobenzene sulfonic acid | 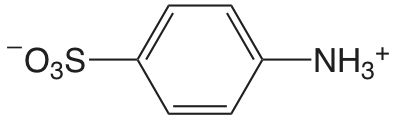 |
3.232 | \(5.86 \times 10^{-4}\) |
| 2-aminobenzoic acid | 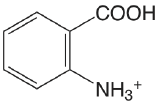 |
2.08 (\(\ce{COOH}\)) 4.96 (\(\ce{NH3}\)) |
\(8.3 \times 10^{-3}\) \(1.1 \times 10^{-5}\) |
| 2-aminophenol (\(T = 20 \text{°C}\)) | 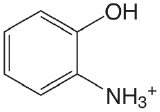 |
4.78 (\(\ce{NH3}\)) 9.97 (OH) |
\(1.7 \times 10^{-5}\) \(1.05 \times 10^{-10}\) |
| ammonia | \(\ce{NH4+}\) | 9.244 | \(5.70 \times 10^{-10}\) |
| arginine | 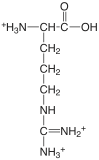 |
1.823 (COOH) 8.991 (\(\ce{NH3}\)) [12.48] (\(\ce{NH2}\)) |
\(1.50 \times 10^{-2}\) \(1.02 \times 10^{-9}\) [\(3.3 \times 10^{-13}\)] |
| arsenic acid | \(\ce{H3AsO4}\) |
2.24 6.96 11.50 |
\(5.8 \times 10^{-3}\) \(1.1 \times 10^{-7}\) \(3.2 \times 10^{-12}\) |
| asparagine (\(\mu = 0.1 \text{ M}\)) |  |
2.14 (COOH) 8.72 (\(\ce{NH3}\)) |
\(7.2 \times 10^{-3}\) \(1.9 \times 10^{-9}\) |
| aspartic acid | 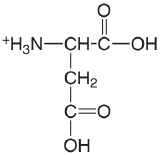 |
1.990 (\(\alpha\)-COOH) 3.900 (\(\beta\)-COOH) 10.002 (\(\ce{NH3}\)) |
\(1.02 \times 10^{-2}\) \(1.26 \times 10^{-4}\) \(9.95 \times 10^{-11}\) |
| benzoic acid | 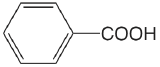 |
4.202 | \(6.28 \times 10^{-5}\) |
| benzylamine | 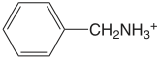 |
9.35 | \(4.5 \times 10^{-10}\) |
| boric acid (\(pK_\text{a2}, pK_\text{a3: } T = 20 \text{°C}\)) | \(\ce{H3BO3}\) |
9.236 [12.74] [13.80] |
\(5.81 \times 10^{-10}\) [\(1.82 \times 10^{-13}\)] [\(1.58 \times 10^{-14}\)] |
| carbonic acid | \(\ce{H2CO3}\) |
6.352 10.329 |
\(4.45 \times 10^{-7}\) \(4.69 \times 10^{-11}\) |
| catechol | 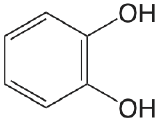 |
9.40 12.8 |
\(4.0 \times 10^{-10}\) \(1.6 \times 10^{-13}\) |
| chloracetic acid | \(\ce{ClCH2COOH}\) | 2.865 | \(1.36 \times 10^{-3}\) |
| chromic acid (\(pK_\text{a1: } T = 20 \text{°C}\)) | \(\ce{H2CrO4}\) |
–0.2 6.51 |
1.6 \(3.1 \times 10^{-7}\) |
| citric acid | 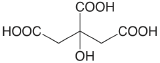 |
3.128 (COOH) 4.761 (COOH) 6.396 (COOH) |
\(7.45 \times 10^{-4}\) \(1.73 \times 10^{-5}\) \(4.02 \times 10^{-7}\) |
| cupferron (\(\mu = 0.1 \text{ M}\)) | 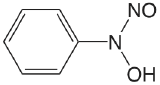 |
4.16 | \(6.9 \times 10^{-5}\) |
| cysteine | 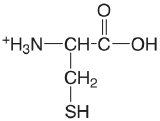 |
[1.71] (COOH) 8.36 (SH) 10.77 (\(\ce{NH3}\)) |
[\(1.9 \times 10^{-2}\)] \(4.4 \times 10^{-9}\) \(1.7 \times 10^{-11}\) |
| dichloracetic acid | \(\ce{Cl2CHCOOH}\) | 1.30 | \(5.0 \times 10^{-2}\) |
| diethylamine | \(\ce{(CH3CH2)2NH2+}\) | 10.933 | \(1.17 \times 10^{-11}\) |
| dimethylamine | \(\ce{(CH3)2NH2+}\) | 10.774 | \(1.68 \times 10^{-11}\) |
| dimethylglyoxime | 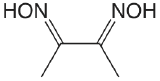 |
10.66 12.0 |
\(2.2 \times 10^{-11}\) \(1. \times 10^{-12}\) |
| ethylamine | \(\ce{CH3CH2NH3+}\) | 10.636 | \(2.31 \times 10^{-11}\) |
| ethylenediamine | \(\ce{+H3NCH2CH2NH3+}\) |
6.848 9.928 |
\(1.42 \times 10^{-7}\) \(1.18 \times 10^{-10}\) |
|
ethylenediaminetetracetic acid (EDTA) (\(\mu = 0.1 \text{ M}\)) |
 |
0.0 (COOH) 1.5 (COOH) 2.0 (COOH) 2.66 (COOH) 6.16 (NH) 10.24 (NH) |
1.0 \(3.2 \times 10^{-2}\) \(1.0 \times 10^{-2}\) \(2.2 \times 10^{-3}\) \(6.9 \times 10^{-7}\) \(5.8 \times 10^{-11}\) |
| formic acid | \(\ce{HCOOH}\) | 3.745 | \(1.80 \times 10^{-4}\) |
| fumaric acid | 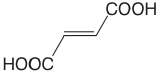 |
3.053 4.494 |
\(8.85 \times 10^{-4}\) \(3.21 \times 10^{-5}\) |
| glutamic acid | 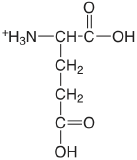 |
2.33 (\(\alpha\)-COOH) 4.42 (\(\lambda\)-COOH) 9.95 (\(\ce{NH3}\)) |
\(5.9 \times 10^{-3}\) \(3.8\times 10^{-5}\) \(1.12 \times 10^{-10}\) |
| glutamine | 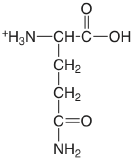 |
2.17 (COOH) 9.01 (\(\ce{NH3}\)) |
\(6.8 \times 10^{-3}\) \(9.8 \times 10^{-10}\) |
| glycine | 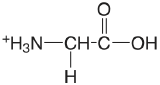 |
2.350 (COOH) 9.778 (\(\ce{NH3}\)) |
\(4.47 \times 10^{-3}\) \(1.67 \times 10^{-10}\) |
| glycolic acid | \(\ce{HOOCH2COOH}\) |
3.881 (COOH) |
\(1.48 \times 10^{-4}\) |
| histidine (\(\mu = 0.1 \text{ M}\)) | 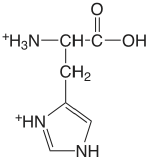 |
1.7 (COOH) 6.02 (NH) 9.08 (\(\ce{NH3}\)) |
\(2. \times 10^{-2}\) \(9.5 \times 10^{-7}\) \(8.3 \times 10^{-10}\) |
| hydrogen cyanide | \(\ce{HCN}\) | 9.21 | \(6.2 \times 10^{-10}\) |
| hydrogen fluroride | \(\ce{HF}\) | 3.17 | \(6.8 \times 10^{-4}\) |
| hydrogen peroxide | \(\ce{H2O2}\) | 11.65 | \(2.2 \times 10^{-12}\) |
| hydrogen sulfide | \(\ce{H2S}\) |
7.02 13.9 |
\(9.5 \times 10^{-8}\) \(1.3 \times 10^{-14}\) |
| hydrogen thiocyanate | \(\ce{HSCN}\) | 0.9 | \(1.3 \times 10^{-1}\) |
| 8-hydroxyquinoline | 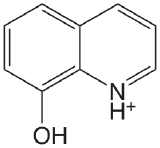 |
4.9 (NH) 9.81 (OH) |
\(1.2 \times 10^{-5}\) \(1.6 \times 10^{-10}\) |
| hydroxylamine | \(\ce{HONH3+}\) | 5.96 | \(1.1 \times 10^{-6}\) |
| hypobromous acid | \(\ce{HOBr}\) | 8.63 | \(2.3 \times 10^{-9}\) |
| hypochlorous acid | \(\ce{HOCl}\) | 7.53 | \(3.0\times 10^{-8}\) |
| hypoiodous acid | \(\ce{HOI}\) | 10.64 | \(2.3 \times 10^{-11}\) |
| iodic acid | \(\ce{HIO3}\) | 0.77 | \(1.7 \times 10^{-1}\) |
| isoleucine | 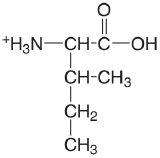 |
2.319 (COOH) 9.754 (\(\ce{NH3}\)) |
\(4.8 \times 10^{-3}\) \(1.76 \times 10^{-10}\) |
| leucine | 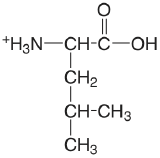 |
2.329 (COOH) 9.747 (\(\ce{NH3}\)) |
\(4.69 \times 10^{-3}\) \(1.79 \times 10^{-10}\) |
| lysine (\(\mu = 0.1 \text{ M}\)) | 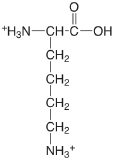 |
2.04 (COOH) 9.08 (\(\alpha \text{-} \ce{NH3}\)) 10.69 (\(\epsilon \text{-} \ce{NH3}\)) |
\(9.1 \times 10^{-3}\) \(8.3 \times 10^{-10}\) \(2.0 \times 10^{-11}\) |
| maleic acid | 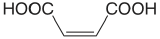 |
1.910 6.332 |
\(1.23 \times 10^{-2}\) \(4.66 \times 10^{-7}\) |
| malic acid | 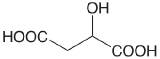 |
3.459 (COOH) 5.097 (COOH) |
\(3.48 \times 10^{-4}\) \(8.00 \times 10^{-6}\) |
| malonic acid | \(\ce{HOOCCH2COOH}\) |
2.847 5.696 |
\(1.42 \times 10^{-3}\) \(2.01 \times 10^{-6}\) |
| methionine (\(\mu = 0.1 \text{ M}\)) | 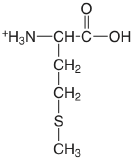 |
2.20 (COOH) 9.05 (\(\ce{NH3}\)) |
\(6.3 \times 10^{-3}\) \(8.9 \times 10^{-10}\) |
| methylamine | \(\ce{CH3NH3+}\) | 10.64 | \(2.3 \times 10^{-11}\) |
| 2-methylanaline | 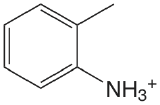 |
4.447 | \(3.57 \times 10^{-5}\) |
| 4-methylanaline | 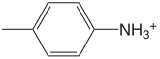 |
5.084 | \(8.24 \times 10^{-6}\) |
| 2-methylphenol | 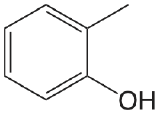 |
10.28 | \(5.2 \times 10^{-11}\) |
| 4-methylphenol | 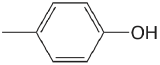 |
10.26 | \(5.5 \times 10^{-11}\) |
|
nitrilotriacetic acid (\(T = 20 \text{°C}), pK_\text{a1: } \mu = 0.1 \text{ M}\)) |
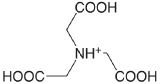 |
1.1 (COOH) 1.650 (COOH) 2.940 (COOH) 10.334 (\(\ce{NH3}\)) |
\(8. \times 10^{-2}\) \(2.24 \times 10^{-2}\) \(1.15 \times 10^{-3}\) \(4.63 \times 10^{-11}\) |
| 2-nitrobenzoic acid | 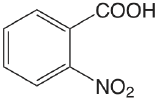 |
2.179 | \(6.62 \times 10^{-3}\) |
| 3-nitrobenzoic acid | 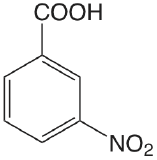 |
3.449 | \(3.56 \times 10^{-4}\) |
| 4-nitrobenzoic acid | 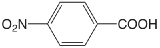 |
3.442 | \(3.61 \times 10^{-4}\) |
| 2-nitrophenol | 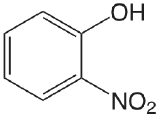 |
7.21 | \(6.2 \times 10^{-8}\) |
| 3-nitrophenol | 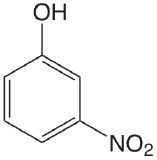 |
8.39 | \(4.1 \times 10^{-9}\) |
| 4-nitrophenol | 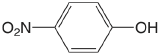 |
7.15 | \(7.1 \times 10^{-8}\) |
| nitrous acid | \(\ce{HNO2}\) | 3.15 | \(7.1 \times 10^{-4}\) |
| oxalic acid | \(\ce{H2C2O4}\) |
1.252 4.266 |
\(5.60 \times 10^{-2}\) \(5.42 \times 10^{-5}\) |
| 1,10-phenanthroline | 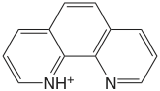 |
4.86 | \(1.38 \times 10^{-5}\) |
| phenol | 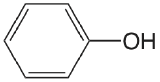 |
9.98 | \(1.05 \times 10^{-10}\) |
| phenylalanine | 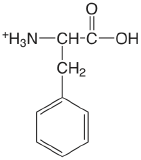 |
2.20 (COOH) 9.31 (\(\ce{NH3}\)) |
\(6.3 \times 10^{-3}\) \(4.9 \times 10^{-10}\) |
| phosphoric acid | \(\ce{H3PO4}\) |
2.148 7.199 12.35 |
\(7.11 \times 10^{-3}\) \(6.32 \times 10^{-8}\) \(4.5 \times 10^{-13}\) |
| phthalic acid | 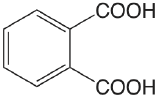 |
2.950 5.408 |
\(1.12 \times 10^{-3}\) \(3.91 \times 10^{-6}\) |
| piperdine | 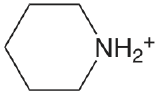 |
11.123 | \(7.53 \times 10^{-12}\) |
| proline |  |
1.952 (COOH) 10.650 (NH) |
\(1.12 \times 10^{-2}\) \(2.29 \times 10^{-11}\) |
| propanoic acid | \(\ce{CH3CH2COOH}\) |
4.874 |
\(1.34 \times 10^{-5}\) |
| propylamine | \(\ce{CH3CH2CH2NH3+}\) | 10.566 | \(2.72 \times 10^{-11}\) |
| pyridine | 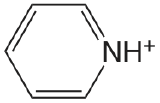 |
5.229 | \(5.90 \times 10^{-6}\) |
| resorcinol | 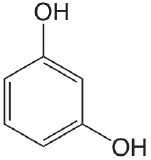 |
9.30 11.06 |
\(5.0 \times 10^{-10}\) \(8.7 \times 10^{-12}\) |
| salicylic acid | 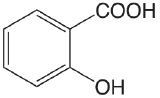 |
2.97 (COOH) 13.74 (OH) |
\(1.1 \times 10^{-3}\) \(1.8 \times 10^{-14}\) |
| serine | 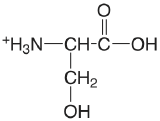 |
2.187 (COOH) 9.209 (\(\ce{NH3}\)) |
\(6.50 \times 10^{-3}\) \(6.18 \times 10^{-10}\) |
| succinic acid |  |
4.207 5.636 |
\(6.21 \times 10^{-5}\) \(2.31 \times 10^{-6}\) |
| sulfuric acid | \(\ce{H2SO4}\) |
strong 1.99 |
— \(1.0 \times 10^{-2}\) |
| sulfurous acid | \(\ce{H2SO3}\) |
1.91 7.18 |
\(1.2 \times 10^{-2}\) \(6.6 \times 10^{-8}\) |
| D-tartaric acid | 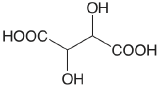 |
3.036 (COOH) 4.366 (COOH) |
\(9.20 \times 10^{-4}\) \(4.31 \times 10^{-5}\) |
| threonine | 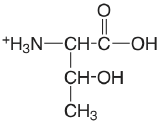 |
2.088 (COOH) 9.100 (\(\ce{NH3}\)) |
\(8.17 \times 10^{-3}\) \(7.94 \times 10^{-10}\) |
| thiosulfuric acid | \(\ce{H2S2O3}\) |
0.6 1.6 |
\(3. \times 10^{-1}\) \(3. \times 10^{-2}\) |
| trichloracetic acid (\(\mu = 0.1 \text{ M}\)) | \(\ce{Cl3CCOOH}\) | 0.66 | \(2.2 \times 10^{-1}\) |
| triethanolamine | \(\ce{(HOCH2CH2)3NH+}\) | 7.762 | \(1.73 \times 10^{-8}\) |
| triethylamine | \(\ce{(CH3CH2)3NH+}\) | 10.715 | \(1.93 \times 10^{-11}\) |
| trimethylamine | \(\ce{(CH3)3NH+}\) | 9.800 | \(1.58 \times 10^{-10}\) |
| tris(hydroxymethyl)amino methane (TRIS or THAM) | \(\ce{(HOCH2)3CNH3+}\) | 8.075 | \(8.41 \times 10^{-9}\) |
| tryptophan (\(\mu = 0.1 \text{ M}\)) | 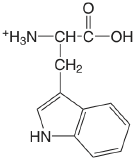 |
2.35 (COOH) 9.33 (\(\ce{NH3}\)) |
\(4.5 \times 10^{-3}\) \(4.7 \times 10^{-10}\) |
| tyrosine (\(pK_\text{a1: } \mu = 0.1 \text{ M}\)) |  |
2.17 (COOH) 9.19 (\(\ce{NH3}\)) 10.47 (OH) |
\(6.8 \times 10^{-3}\) \(6.5 \times 10^{-10}\) \(3.4 \times 10^{-11}\) |
| valine | 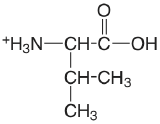 |
2.286 (COOH) 9.718 (\(\ce{NH3}\)) |
\(5.18 \times 10^{-3}\) \(1.91 \times 10^{-10}\) |

