2.6: Arrangements of Electrons
- Page ID
- 83053
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Skills to Develop
- To describe how electrons are grouped within atoms.
Although we have discussed the general arrangement of subatomic particles in atoms, we have said little about how electrons occupy the space about the nucleus. Do they move around the nucleus at random, or do they exist in some ordered arrangement?
The modern theory of electron behavior is called quantum mechanics. It makes the following statements about electrons in atoms:
- Electrons in atoms can have only certain specific energies. We say that the energies of the electrons are quantized.
- Electrons are organized according to their energies into sets called shells. Generally the higher the energy of a shell, the farther it is (on average) from the nucleus. Shells do not have specific, fixed distances from the nucleus, but an electron in a higher-energy shell will spend more time farther from the nucleus than does an electron in a lower-energy shell.
- Shells are further divided into subsets of electrons called subshells. The first shell has only one subshell, the second shell has two subshells, the third shell has three subshells, and so on. The subshells of each shell are labeled, in order, with the letters s, p, d, and f. Thus, the first shell has only an s subshell, the second shell has an s and a p subshell, the third shell has s, p, and d subshells, and so forth.
- Different subshells hold a different maximum number of electrons. Any s subshell can hold up to 2 electrons; p supshell up to 6 electrons; d subshell up to 10; and f subshell up to 14.
It is the arrangement of electrons into shells that has the most effect on chemical properties, so we will focus on mainly on shells here.
We use numbers to indicate which shell an electron is in. The first shell, closest to the nucleus and with the lowest-energy electrons, is shell 1. This first shell has only one subshell (labeled 1s) and can hold a maximum of 2 electrons. This is why there are two elements in the first row of the periodic table (H & He).
Because the first shell can only hold a maximum of 2 electrons, the third electron must go into the second shell. Therefore the lithium (Li), which has three total electrons, will have two electrons in the first shell and one electron in the second shell. Notice that lithium is the first element in the second row of the periodic table.
The second shell has two subshells (labeled 2s and 2p). The 2s subshell holds a maximum of 2 electrons, and the 2p subshell holds a maximum of 6 electrons. This means that the second shell can hold a maximum of eight electrons (2+6=8). Notice that there are eight elements in the second row of the periodic table.
It is only the electrons in the outer-most shell, called the VALENCE shell, that tend to react (be gained, lost, or shared). You might imagine that, if two atoms bumped into each other, it would be the outer electrons that would interact first. The following is a list of total electrons, electrons by shell, and valence electrons for the first 10 elements.
- Hydrogen has 1 electron in the first shell (so one valence electron).
- Helium has 2 electrons --- both in the first shell (so two valence electrons).
- Lithium has 3 electrons --- 2 in the first shell, and 1 in the second shell (so one valence electron).
- Beryllim has 4 electrons --- 2 in the first shell, and 2 in the second shell (so two valence electrons).
- Boron has 5 electrons --- 2 in the first shell, and 3 in the second shell (so three valence electrons).
- Carbon has 6 electrons --- 2 in the first shell, and 4 in the second shell (so four valence electrons).
- Nitrogen has 7 electrons --- 2 in the first shell, and 5 in the second shell (so five valence electrons).
- Oxygen has 8 electrons --- 2 in the first shell, and 6 in the second shell (so six valence electrons).
- Fluorine has 9 electrons --- 2 in the first shell, and 7 in the second shell (so seven valence electrons).
- Neon has 10 electrons --- 2 in the first shell, and 8 in the second shell (so eight valence electrons).
Figure 2.6.1 below lists the atomic number for the main group elements. The atomic number defines the number of protons in the nucleus of each atom. For neutral atoms, the number of positive protons will equal the total number of negative electrons (zero net charge). For example, bromine (Br) has 35 protons and 35 total electrons. Periodic tables always list the atomic number.
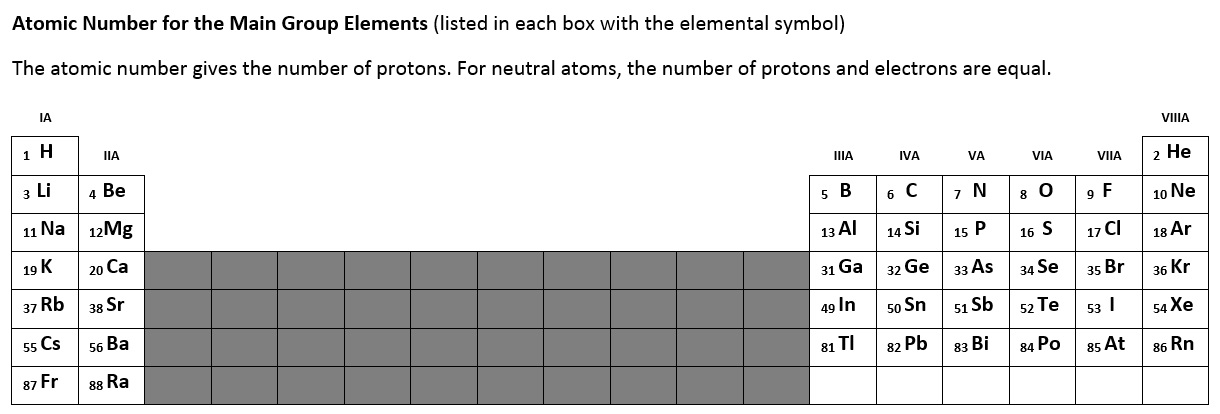
Figure 2.6.1 - Atomic Number for Each of the Main Group Elements
The number of valence electrons for each main group element can be determined by the column, or group, it occupies on the periodic table. Table 2.6.2 below summarizes the number of valence electrons for each main group column of elements. For example, the elements in the first column (sometimes labeled IA), all have one valence electron. The second column (IIA) has two valence electrons. We skip the short block of ten elements in the middle because this is where a subshell fills out of order. The elements in columns IIIA, IVA, VA, VIA, and VIIA, and VIIIA* have three, four, five, six, seven, and eight* valence electrons, respectively.
*Note that helium (He) only has two valence electrons. Some periodic tables place helium in column IIA, others place it in VIIIA, and some in both locations.
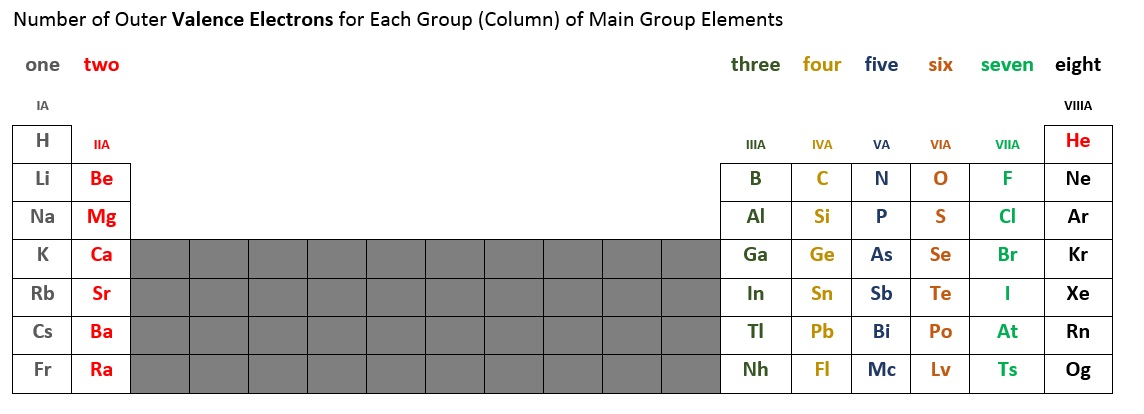
Figure 2.6.2 - Number of Valence Electrons for Main Group Elements
Example \(\PageIndex{1}\): Electrons of Phosphorus
How many total and valence electrons are in a neutral phosphorus atom?
SOLUTION
A neutral phosphorus atom has 15 total electrons. Two electrons can go into first shell, eight in the second shell, and it has five more in the third shell. The third shell is the outer valence shell, so it has 5 valence electrons.
The number of electrons in each shell becomes more complicated as more electrons are added because there are more subshells being used and because the shell start to fill out of order. For elements with larger atomic number than 20 (beyond calcium), we will just focus on how many total and how many valence electrons, not the number in each shell. We have stated that the outer-shell electrons are called valence. The inner (non-valence) shells and electrons are often called the core.
Example \(\PageIndex{2}\): Counting total and Valence Electrons in Xenon
How many total, valence, and core electrons are there in a neutral xenon atom?
SOLUTION
Xenon has 54 total, 8 valence, and 46 core electrons.
Concept Review Exercises
- How are electrons organized in atoms?
- What is the maximum number of electrons that can fit into the first two shells of an atom?
- What is the difference between core electrons and valence electrons?
Answers
- Electrons are organized into shells and subshells around nuclei.
- The first shell can fit a maximum of two and the second shell can fit a maximum of eight electrons.
- Valence electrons are in the highest-numbered (outer) shell; all other electrons are core electrons.
Key Takeaway
- Electrons are organized into shells and subshells about the nucleus of an atom.
Contributors
Anonymous

