23.1 Carbonyl Condensations - The Aldol Reaction
- Page ID
- 91014
Objectives
After completing this section, you should be able to
- write a general mechanism for carbonyl condensation reactions.
- write an equation to illustrate the aldol condensation reaction.
- identify the product formed when an aldehyde or ketone having an alpha‑hydrogen atom is treated with base in a protic medium.
- identify the aldehyde or ketone, and other reagents required to produce a given β‑hydroxy carbonyl compound by an aldol reaction.
- determine whether a given aldehyde or ketone will undergo an aldol reaction.
- write the detailed mechanism of the aldol reaction.
- aldol
- aldol reaction
- carbonyl condensation reaction (see Chapter 18 Affix)
It is important that you understand the general mechanism of carbonyl condensation described in this section: once you grasp this mechanism, you will see that all the reactions that follow are very similar.
The aldol reaction is sometimes referred to as the aldol condensation. However, a condensation reaction is often regarded as a reaction in which two molecules join together with the elimination of a molecule of water (or some other compound of low molar mass). Thus, the aldol reaction described here is not a true condensation; the true aldol condensation is described later, in Section 23.3. It is perhaps unfortunate that the reactions discussed in this unit are all described as condensation reactions whether or not water is eliminated.
The term “aldol” (from "aldehyde alcohol") is used both to describe the specific compound 3‑hydroxybutanal:

and to describe β‑hydroxy aldehydes in general.
A useful carbon-carbon bond-forming reaction known as the Aldol Reaction is yet another example of electrophilic substitution at the alpha carbon in enolate anions. The fundamental transformation in this reaction is a dimerization of an aldehyde (or ketone) to a beta-hydroxy aldehyde (or ketone) by alpha C–H addition of one reactant molecule to the carbonyl group of a second reactant molecule. Due to the carbanion like nature of enolates they can add to carbonyls in a similar manner as Grignard reagents. For this reaction to occur at least one of the reactants must have α hydrogens.
General Aldol reaction
Going from reactants to products simply
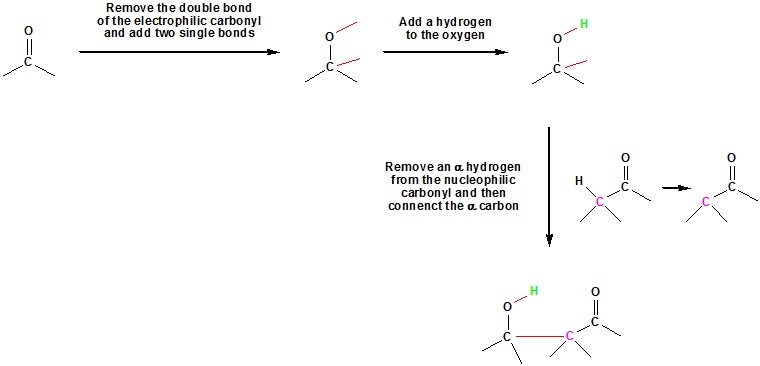
Aldol Reaction Mechanism
Step 1: Enolate formation
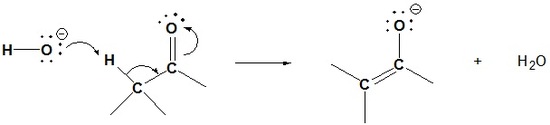
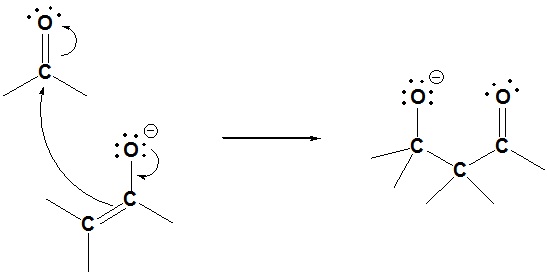
Step 2: Nucleophilic attack by the enolate
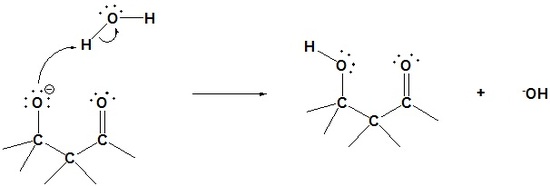
Step 3: Protonation
What would be the expect product of the following aldol reaction?
- Answer
-
Analysis:
When considering the product of an aldol reaction it is vital to consider each reactant molecule separately. Also,
Identify electrophilic carbonyl carbon and any alpha hydrogens present.
Exercises
Predict the product of an aldol reaction with the following molecules:
a)
b)
c)
- Answer
-
a)
b)
c)
Because the aldol reaction is reversible it is possible for a beta-hydroxy carbonyl compound to undergo a retro-aldol reaction. Please draw the mechanism or the based catalyzed retro-aldol reaction shown below.
- Answer
-
Contributors and Attributions
Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)
Prof. Steven Farmer (Sonoma State University)

