15.4: Aromatic Ions
- Page ID
- 67318
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
Objectives
After completing this section, you should be able to
- use the Hückel 4n + 2 rule to explain the stability of the cyclopentadienyl anion, the cycloheptatrienyl cation and similar species.
- use the Hückel 4n + 2 rule to determine whether or not a given unsaturated cyclic hydrocarbon anion or cation is aromatic.
- draw the resonance contributors for the cyclopentadienyl anion, cation and radical, and similar species.
Charged Aromatic Compounds
Carbanions and carbocations may also show aromatic stabilization. Some examples are:
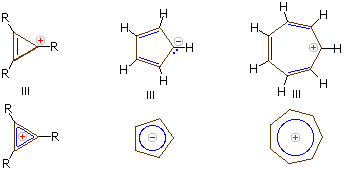
The three-membered ring cation has 2 \(\pi\)-electrons and is surprisingly stable, considering its ring strain. Cyclopentadiene is as acidic as ethanol, reflecting the stability of its 6 π-electron conjugate base. Salts of cycloheptatrienyl cation (tropylium ion) are stable in water solution, again reflecting the stability of this 6 \(\pi\)-electron cation.
Exercises
Draw the resonance structures for cycloheptatriene anion. Are all bonds equivalent? How many lines (signals) would you see in a H1 NMR?
- Answer
-
All protons and carbons are the same, so therefore each spectrum will only have one signal in the proton NMR.
The following reaction occurs readily. Propose a reason why this occurs?
- Answer
-
The ring becomes aromatic with the addition of two electrons. Thereby obeying the 4n+2 rule.
Contributors and Attributions
Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)
Prof. Steven Farmer (Sonoma State University)
William Reusch, Professor Emeritus (Michigan State U.), Virtual Textbook of Organic Chemistry

