Exercises: Main Group Chemistry
- Page ID
- 40710
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Periodic Trends
1. The most accurate definition of ionization energy is ?
a) the energy required to add an electron to an atom or ion,
b) the energy required for an atom or ion to form a bond,
c) the energy required to remove a proton from the nucleus of an atom or ion,
and d) the energy required to remove an electron from an atom or ion
2. The most accurate definition of the term "electron affinity" is ?
a) the amount of energy released when an atom forms a bond,
b) the amount of energy released when an electron is lost from a neutral atom to form a cation,
c) the amount of energy required for a neutral atom to form a anion,
and d) the amount of energy released when an electron is added to a neutral atom to form an anion
3. Place the following elements in order of increasing electron affinity: Cl, Na, O, C.
a) Na, C, O, Cl,
b) Cl, O, C, Na,
c) Na, C, Cl, O,
and d) Na, O, C, Cl
4. Rank the following elements in order of increasing atomic radius: Na, B, F, Br
a) Br < Na < B < F,
b) F < B < Br < Na,
c) B < F < Na < Br,
and d) F < B < Na < Br
5. The largest atomic radius for a neutral element is expected for which of the following electron configurations?
a) [He] 2s2 2p3,
b) [Ne] 3s2 3p3,
c) [Ne] 3s2 3p5,
and d) [He] 2s2 2p5
6. Which of the following is expected to have the smallest crystal ionic radius?
a) Li+,
b) Ca2+,
c) P3+,
and d) Al3+
Short Answer Questions
- Describe the contributions of de Chancourtois, Newlands and Mendeleev to the development of the modern periodic table.
- 2. Calculate the effective nuclear charge for i) an outer p electron in an oxygen atom and ii) a 3d electron in a nickel atom iii) a 4s electron in a nickel atom.
- What is the main difference between electron affinity and electronegativity?
- Which do you predict to have the greater electron affinity, Y or Y- ? Why?
- Predict the type of bond that would form between elements with χ = 2.5 and 4.0? (Pauling scale).Would this bond be more or less polar than a N-H bond? Justify your answer.
- Account for the general increase in ionization energy across period 3.
- Account for the dip in ionization energy that is observed from Mg to Al and from P to S.
- How might the ionization energy, electron affinity and electronegativity affect the reactivity of the elements?
- The molecules NH3 and NF3 have trigonal pyramidal structures, and dipole moments of 1.47 and 0.24D respectively. The values of χP(N), χP(H) and χP(F) are 3.0, 2.2 and 4.0. Explain the variation in the observed dipole moments and show the direction of the dipoles.
Hydrogen
Short Answer Questions
- Hydrogen has only 1 proton but its first ionization energy is much greater than that of lithium, which has three protons. Explain.
- Outline the differences between ortho- and para- hydrogen. How can you isolate each of these forms in a pure state?
- How can you prepare hydrogen in the laboratory?
- Where is the best location for hydrogen in the Periodic Table, at the top of Group 1 or Group 17?
- Account for the fact that the Alkali metal hydrides are much less stable than their chlorides.
- Show how metal hydride compounds can be used in synthetic Organic chemistry reactions, e.g for treating carbonyls and esters.
- Classify NaH, CH4 and HCl as covalent or saline hydrides and explain how the reaction of each of these with water illustrates different aspects of the chemistry of hydrogen.
- The boiling points of the hydrogen halides follow the trend: HF(20 °C) > HCl (-85 °C) < HBr(-67 °C) < HI(-36 °C). Explain.
- Place the following series of compounds in order of increasing covalent character:
i) BeCl2, BI3, LiF.
ii) Na2S, NaCl, Na3P.
Explain your reasoning.
Oxygen
Muliple Choice Questions
1. In which of the following reactions is H2O2 not acting as an oxidizing agent?
a) 2Fe2+ + H2O2 + 2H+ → 2Fe3+ + 2H2O
b) PbS + 4H2O2 → PbSO4 + 4H2O
c) 2I- + 2H2O2 + 2H+ → I2 + 2H2O
d) Mn2+ + H2O2 + 2OH- → MnO2 + 2H2O
e) Ag2O + 2H2O2 → 2Ag + O2 + H2O
Short Answer Questions
- You are provided with two liquids, one is H2O and the other is H2O2. Safety regulations now prohibit you from touching/tasting them so suggest chemical tests that could distinguish between them. Include the reagents and observations expected and show balanced equations for the reactions.
- Use the MO diagram below to determine the bond order for the following species and predict whether the bond lengths shown are consistent with your values.
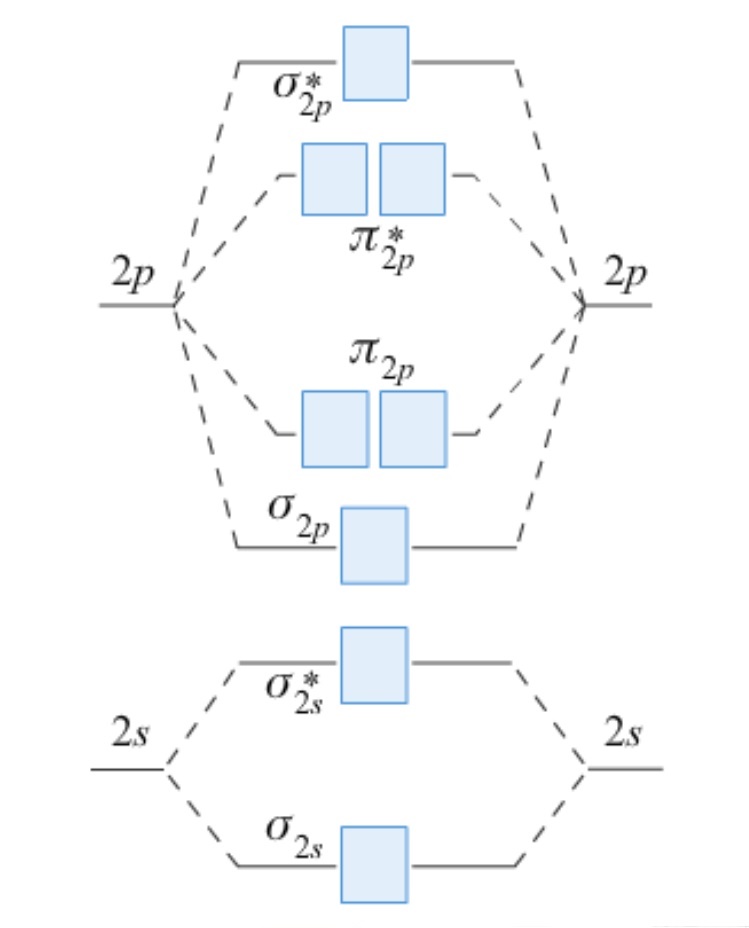
O2+ = 112 pm
O2 = 121 pm
O2- = 128 pm
O22- = 149 pm - With the aid of suitable equations describe how H2O2 can be produced industrially and on a small scale in the laboratory.
- Write balanced half equations and the net ionic equation for:
i) the oxidation of Fe2+ by H2O2 in acidic solution.
ii) the reaction between KMnO4 and H2O2 in acidic solution.
iii) the reaction between acidified KBr and H2O2. - Describe two uses of D2O, including the chief large scale use.
- With the aid of equations, describe how ozone is produced and destroyed in the upper atmosphere.
- How would you prepare ozone in the laboratory? What conditions and safety equipment would be needed?
- What experiment/techniques could be used to distinguish between O3 and O2?
- How does the O-O bond length in O3 of 127.2 pm compare to the data above for O2 species?
Structure of elements and Acid/bases
Multiple Choice Questions
1. Which of the following statements is correct with regard to the reaction:
a) HNO3 acts as a Lewis acid
b) HNO3 acts as an oxidising agent
c) HF acts as a Lewis acid
d) HF acts as a Lewis base
Short Answer Questions
- Using suitable diagrams describe the difference between the following terms:
i) hexagonal close packing (hcp) and cubic close packing (ccp).
ii) octahedral holes and tetrahedral holes in close-packed structures. - Why should white phosphorus (MP 44 °C) be less stable than red phosphorus (MP 590 °C) ?
- What do the structures of black phosphorus and graphite have in common? Use diagrams to illustrate.
- Sketch the structures of 3 allotropes of sulfur
- Write balanced equations for the reaction between water and each of the following and state whether the resulting solution is acidic or basic.
i) K2O ii) SO3 iii) NH3 - Describe the structure of water and ice. Account for the differences between the two.
- Explain the relation between the fact that water expands on freezing, and the fact it has a much higher BP that all other group 16 hydrides.
- Outline the differences and similarities between the various concepts used to define acids and bases.
Coordination
MCQ
- What is the electronic configuration for a Cu(II) ion?:
d8, d10, d7, d9, none of these - What is the electronic configuration for a Mn(II) ion?:
d6, d5, d7, d4, none of these - What is the electronic configuration for a Co(II) ion?:
d7, d5, d8, d6, none of these - What is the electronic configuration for a Fe(III) ion?:
d6, d4, d7, d5, none of these - What is the electronic configuration for a Cr(II) ion?:
d5, d3, d2, d4, none of these - What is the electronic configuration for a Ti(III) ion?:
d0, d1, d2, d3, none of these - Which of the following electronic configurations is correct?:
Ni(II)-d8, Fe(III)-d5, V(V)-d1,
Co(II)-d8, Fe(II)-d6, Cr(II)-d4,
Cr(III)-d3, Ti(III)-d1, Mn(VII)-d0,
Fe(III)-d5, Ni(II)-d9, Cu(I)-d10,
none of these - Which of the following electronic configurations is correct?:
Mn(II)-d5,Cu(I)-d9, Co(II)-d7,
Co(III)-d6, Cu(II)-d9, Cr(II)-d4,
Fe(II)-d6, Ti(III)-d0, V(IV)-d1,
V(III)-d3, Fe(III)-d5, Mn(IV)-d3,
none of these
1). (a) Give the Oxidation number, d-orbital occupation, co-ordination number and expected magnetic moment of the central metal ion in the following complexes.
Draw the expected structure.
(i) K3[Co(C2O4)3]
(ii) (NH4)2[CoF4]
(iii) diamagnetic [NiCl2{P(C6H5)3}2]
(iv) cis-[CrCl2(bipy)2]Cl
(v) [Mn(H2O)6]SO4
where C2O42- is the oxalate ion and bipy is 2,2'-bipyridine.
(b) Which of the complexes above can exhibit isomerism? Explain.
(c) Give the IUPAC name for the complex (ii) in part (a).
Answers
2) Write down the systematic name for each of the following complexes and indicate the coordination number, oxidation number, electronic configuration, stereochemistry and magnetic moment of the central ion.
- K[Cr(oxal)2(H2O)2].3H2O
- CrCl3(py)3
- K4[Mn(CN)6]
- [CoCl(NH3)5]Cl2
- Cs[FeCl4]
- [NiCl(NH3)(en)2]Cl
- [Cu(NH3)4(H2O)]SO4
Answers
3) The hexaquo manganese(II) ion contains five unpaired electrons, while the hexacyano- ion contains only one unpaired electron. Explain, using Crystal Field Theory.
Answers
4) Indicate the type of isomerism exhibited by the following complexes and draw structures for these isomers.
- K[Cr(oxal)2(H2O)2].3H2O
- [Co(en)3]Cl3
- [CoCl(NO2)(NH3)4]Br
- PtCl2(NH3)(H2O)
Answers
5) Give an example of each of the following:
- Binuclear Complex
- Metal Chelate
- Low spin complex
- High spin complex
- Five coordinate complex.
6) a) Place the following ligands in increasing order in the Spectrochemical series:
CN-, NH3, Cl-,H2O
b) For octahedral first row transition metal complexes with between four and seven d electrons, both high and low spin electron configurations are possible.
Use Crystal Field splitting diagrams to determine the number of unpaired electrons and then calculate the expected spin-only magnetic moments.
7) a) Draw the complex, [Ni(en)3]2+, showing the optical isomers.
b) If at equilibrium, [Ni(en)3]2+, is 0.08M and [en] is 0.40M, calculate [Ni2+].
Note that β3 for [Ni(en)3]2+ is 4.07 x 1018.
c) Write equations for the successive formation equilibria.
d) The first and second stepwise formation constants are:
log K1 = 7.66
and log K2 = 6.40.
Calculate the third stepwise formation constant.
Ans b) 3.07 x 10-19.
d) 3.55 x 104.
8) The complex ion [Ni(NH3)4]2+, forms on mixing aqueous solutions of ammonia and a nickel salt.
a) If a solution contains 1.6 x 10-4 % of the nickel ions in the form of Ni2+ when the concentration of free NH3 (aq) is 0.5M. What is the stability constant of the complex [Ni(NH3)4]2+?
(Assume that this is the only complex present).
b) The octahedral ammine complex can be prepared by using a solution of ammonia which has been supersaturated with ammonia gas, such that:
log K6 = 0.42.
Calculate the overall β6 for [Ni(NH3)6]2+.
Write the equations for the equilibria corresponding to K5 and K6.
Answers
return to course outline
Problems
1a) For the equilibrium given by the reaction:
\[Ni(II) + EDTA^{4-} \leftirightharpoons [Ni(EDTA)]^{2-}\]
the equilibrium constant has been determined to be 3.6 x 1018
Estimate the amount of free Ni(II) in the solution if 0.01M Ni(II) is reacted with 0.11M EDTA.
1b) Calculate the equilibrium concentration of the Cu2+ ion in a solution that is initially 0.10 M Cu2+ and 1.0 M NH3, given that β4 for Cu(NH3)42+= 2.1 x 1013.
This is CALCULATION # ONE from the Chelate Effect Lecture.
2) Calculate the entropy changes for the following reactions at 298K and comment on the results:
(NB R=8.314 J K-1 mol-1)
This is CALCULATION # TWO from the Chelate Effect Lecture.
3) The stepwise enthalpies ΔHn and the stability constants K for the system Ni2+ - en in aqueous solution at 298K are as follows:
| n | log10Kn | ΔH° /kJmol-1 |
|---|---|---|
| 1 | 7.51 | -37.7 |
| 2 | 6.35 | -38.4 |
| 3 | 4.42 | -40.6 |
Calculate the standard free energy ΔG° and entropy changes ΔS° associated with the addition of each ligand.
4) Calculate the overall complex dissociation equilibrium constant for the Cu(NH3)42+ ion given that β4 for this complex is 2.1 x 1013.
5) Calculate the equilibrium concentration of the Fe3+ ion in a solution that is initially 0.10 M Fe3+ and 1.0 M SCN-, given that β2 for Fe(SCN)2+ = 2.3 x 103.
7) What is the ratio of uncomplexed to complexed Zn2+ in a solution that is 10M in NH3 given that β4 for Zn(NH3)42+ = 3 x 109.
8) Given the following data at 25°C:
and ΔG° = -55.6 kJ mol-1 for Ag+(aq) + Cl- <=> AgCl(s)
b) Calculate the equilibrium constant for this reaction.
Will the reaction proceed from L -> R ??

