The Use of Chemical Dispersants for Oil Spills
- Page ID
- 50865
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
The central principle governing solubility is the concept of “like dissolves like.” In other words, molecules with similar intermolecular forces are able to dissolve in each other, while molecules with dramatically different intermolecular forces generally cannot. A classic example is the fact that “oil and water don’t mix.” Oil is composed largely of nonpolar hydrocarbons, which exert only relatively weak London dispersion forces. Water molecules, in contrast, are held together by relatively strong hydrogen bonding between a hydrogen atom on one molecule (which has a partial positive charge) and the oxygen atom on another molecule (which has a partial negative charge).


IWC replica Réplica de reloj The strong intermolecular forces between the water molecules make them tend to cluster together, excluding the more weakly attractive oil molecules, which then group together on their own. On a macro level, this causes water and oil to separate into two different layers. Since oil is less dense than water, the oil layer floats above the water layer, creating an oil “slick” on the surface of the water.

This phenomenon takes on serious environmental consequences when crude oil is spilled into natural bodies of water, whether through an oil tanker accident such as the Exxon Valdez oil spill in 1989, or through an oil well accident such as the BP Deepwater Horizon oil spill in 2010. Overall, the National Research Council estimates that an average of three million gallons of oil and other petroleum products are spilled into U.S. waters every year (1). The sticky, viscous oil slick that forms on the water’s surface can trap and damage seabirds, turtles, and marine mammals, and if washed ashore also threatens biologically important shorelines and beaches (2). While this surface oil can sometimes be removed by skimming or burning, these techniques are not always possible or practical, and have their own potential side effects. (For example, the burning of oil generates carbon dioxide and other airborne pollutants.) An alternative strategy is to try to disperse the oil into the water in small droplets, a process called emulsification. While this does not physically remove the oil from the water, it does break up the potentially dangerous oil slick and prevents it from reaching the shore. Emulsification may also speed up biodegradation of the oil by naturally occurring bacteria by as much as 50% (3).
While the action of wind and waves may naturally disperse some of the oil into small droplets, another approach is to use a chemical dispersant to emulsify the oil. Dispersants tend to be mixtures of three types of chemicals: solvents, additives, and surface-active agents, commonly called surfactants. Surfactants, which are the key to the dispersant’s effectiveness, are compounds containing two different kinds of chemical groups: one that is oil-compatible (lipophilic or hydrophobic) and one that is water-compatible (hydrophilic). The lipophilic end of the surfactant molecule has London dispersion forces similar to those of the oil, allowing this portion of the molecule to “dissolve” in the oil layer. In contrast, the hydrophilic end has dipole-dipole forces and/or hydrogen bonding similar to that of water, allowing this portion of the molecule to “dissolve” in the water. This dual action reduces what is called the interfacial tension between the oil and the water, allowing the oil to break up into small droplets surrounded by surfactant molecules.

Natural water turbulence then pulls these droplets apart and down into the water column (a process called entrainment), allowing the oil to disperse through the water. Note that this does NOT mean that the oil has actually dissolved in the water! The oil and water are still insoluble in each other; however, the dispersant enables the oil to exist in small, stable droplets within the water column instead of in a single layer on top of the water(1).
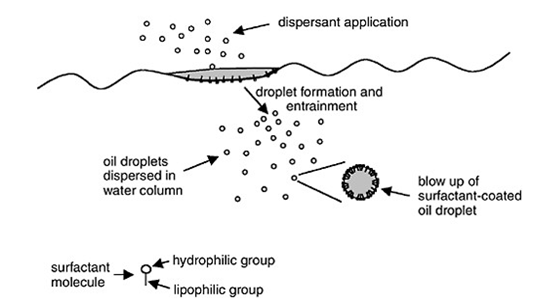
Video showing the action of a dispersant on an oil slick: www.fox10tv.com/dpp/news/gulf...ispersant-work
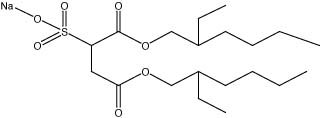
The predominant dispersant used in the BP Deepwater Horizon oil spill was Corexit EC9500A, produced by the Nalco company. The primary surfactants found in this dispersant are sorbitan monooleate and ethoxylated sorbitan monooleates (i.e., surfactants A and B in the diagram above) along with dioctyl sodium sulfosuccinate, which is a detergent that is also commonly found in laxatives (1, 4).
 \
\
Corexit EC9500A has a reasonable dispersant effectiveness of about 55% for South Louisiana crude oil, and the EPA has deemed it no more toxic than other dispersant chemicals currently in use (5, 3). Nevertheless, the application of this dispersant, both in the Deepwater Horizon incident and for other oil spills, remains controversial. As the National Research Council points out, adding a dispersant to an oil spill increases the hydrocarbon load on the ecosystem and has the potential to harm sensitive marine organisms. Thus, the decision to use a dispersant is always a trade-off: it increases the risk to organisms in the water column and the seafloor while decreasing risks to organisms on the water surface and shoreline (1).
From ChemPRIME: 10.18: Solubility and Molecular Structure
References:
1) National Research Council. Oil Spill Dispersants: Efficacy and Effects. Washington, D.C.: National Academies Press, 2005.
2) Graham, P. (2010). Deep Sea Oil Spill Cleanup Techniques: Applicability, Trade-offs and Advantages. ProQuest Discovery Guides, www.csa.com/discoveryguides/oil/review.pdf
3) www.epa.gov/bpspill/dispersants-qanda.html
4) http://www.nytimes.com/gwire/2010/06/09/09greenwire-ingredients-of-controversial-dispersants-used-42891.html
5) www.epa.gov/osweroe1/content/...s/corex950.htm
Contributors and Attributions
Ed Vitz (Kutztown University), John W. Moore (UW-Madison), Justin Shorb (Hope College), Xavier Prat-Resina (University of Minnesota Rochester), Tim Wendorff, and Adam Hahn.


