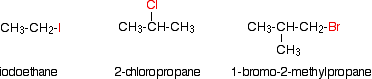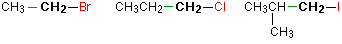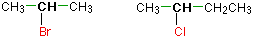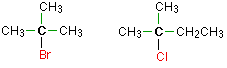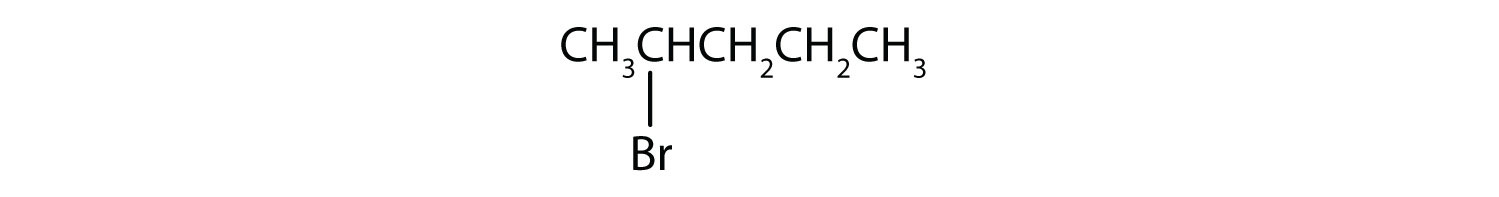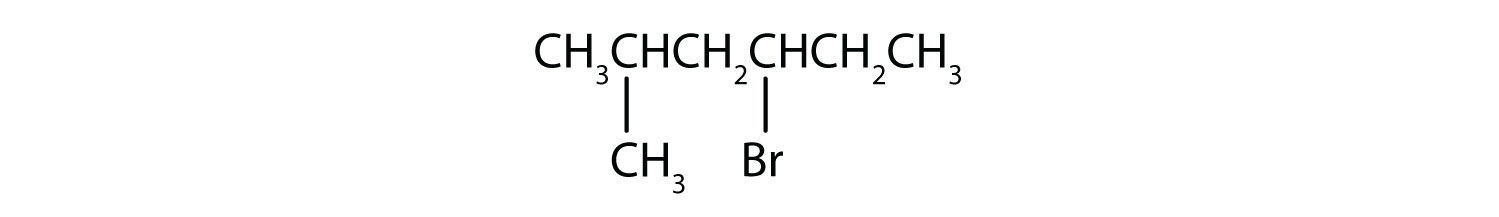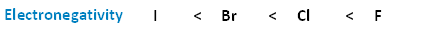Objectives
After completing this section, you should be able to
- write the IUPAC name of a halogenated aliphatic hydrocarbon, given its Kekulé, condensed, or shorthand structure.
- draw the Kekulé, condensed, or shorthand structure of a halogenated aliphatic hydrocarbon, given its IUPAC name.
- write the IUPAC name and draw the Kekulé, condensed, or shorthand structure of a simple alkyl halide, given a systematic, non-IUPAC name (e.g., sec-butyl iodide).
- arrange a given series of carbon-halogen bonds in order of increasing or decreasing length and strength.
Study Notes
This section contains little that is new. If you mastered the IUPAC nomenclature of alkanes, you should have little difficulty in naming alkyl halides. Notice that when a group such as CH2Br must be regarded as a substituent, rather than as part of the main chain, we may use terms such as bromomethyl.
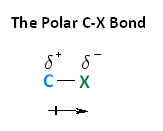
You will find it easier to understand the reactions of the alkyl halides if you keep the polarity of the C-X bond fixed permanently in your mind (see ”The Polar C-X Bond” shown in the reading below).
Alkyl halides are also known as haloalkanes. This page explains what they are and discusses their physical properties. Alkyl halides are compounds in which one or more hydrogen atoms in an alkane have been replaced by halogen atoms (fluorine, chlorine, bromine or iodine). For example:
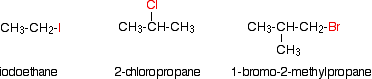
Halide Designations
Alkyl halides fall into different classes depending on how many alkyl groups are attached to the carbon which holds the halogen. There are some chemical differences between the various types. When there are no alkyl groups attached to the carbon holding the halogen, these are considered methyl halides (CH3X).
Primary alkyl halides
In a primary (1°) haloalkane, the carbon which carries the halogen atom is only attached to one other alkyl group. Some examples of primary alkyl halides include:
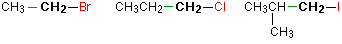
Notice that it doesn't matter how complicated the attached alkyl group is. In each case there is only one linkage to an alkyl group from the CH2 group holding the halogen. There is an exception to this: CH3Br and the other methyl halides are often counted as primary alkyl halides even though there are no alkyl groups attached to the carbon with the halogen on it.
Secondary alkyl halides
In a secondary (2°) haloalkane, the carbon with the halogen attached is joined directly to two other alkyl groups, which may be the same or different. Examples:
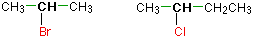
Tertiary alkyl halides
In a tertiary (3°) haloalkane, the carbon atom holding the halogen is attached directly to three alkyl groups, which may be any combination of same or different. Examples:
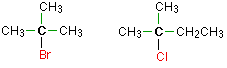
Example \(\PageIndex{1}\)
Please indicate if the following haloalkanes are methyl, 1o, 2o, or 3o:
a) CH3I
b) CH3CH2Br
c)

d)

e)

f)

Solution
a) methyl
b) 1o
c) 2o
d) 3o
e) 1o
f) 2o
Nomenclature of Alkyl Halides
Alkyl halides are systematically named as alkanes (Section 3-4) where the halogen is a substituent on the parent alkane chain. To summarize the rules discussed in detail in Section 3-4, there are three basic steps to naming alkyl halides.
- Find and name the longest carbon chain and name it as the parent chain. Remember is an alkene or alkyne is present, the parent chain must contain both carbons of the multiple bond.
- Number the parent chain consecutively, starting at the end nearest a substituent group. Then assign each substituent a number. Remember the IUPAC system uses a prefix to indicate the halogen followed by the suffix -ide. The prefixes are fluoro- for fluorine, chloro- for chlorine, bromo- from bromine, and iodo- for iodine. The name of a halogen is preceded by a number indicating the substituent’s location on the parent chain.


- If there is an ambiguity in numbering the parent chain, begin on the end which is closer to the substituent which comes first alphabetically.

Common Names of Alkyl Halides
Alkyl halides with simple alkyl groups are often called by common names. Those with a larger number of carbon atoms are usually given IUPAC names. The common names of alkyl halides consist of two parts: the name of the alkyl group plus the first syllable of the name of the halogen, with the ending -ide. The names of common alkyl groups are listed in Section 3.3.

Exercise \(\PageIndex{1}\)
1) Give the common and IUPAC names for each compound.
- CH3CH2CH2Br
- (CH3)2CHCl
- CH3CH2I
- CH3CH2CH2CH2F
2) Give the IUPAC name for each compound.
a)
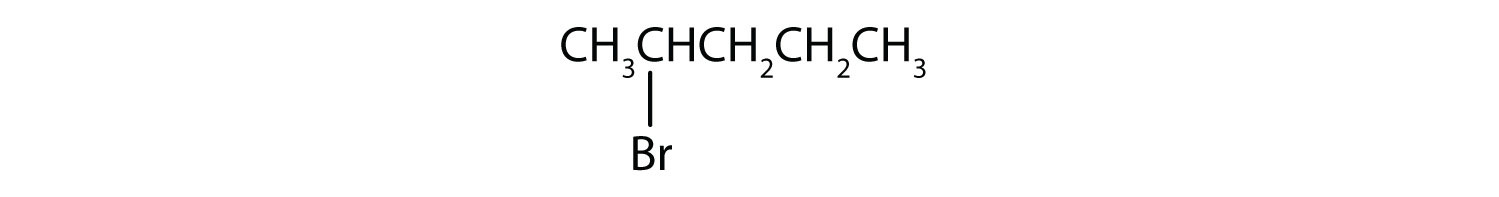
b) 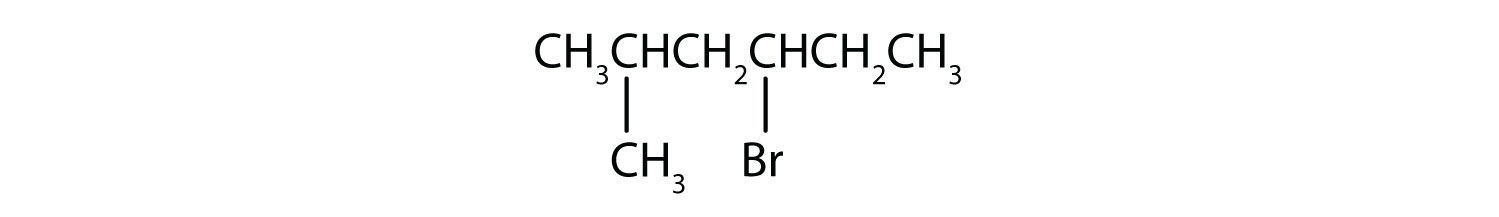
c)
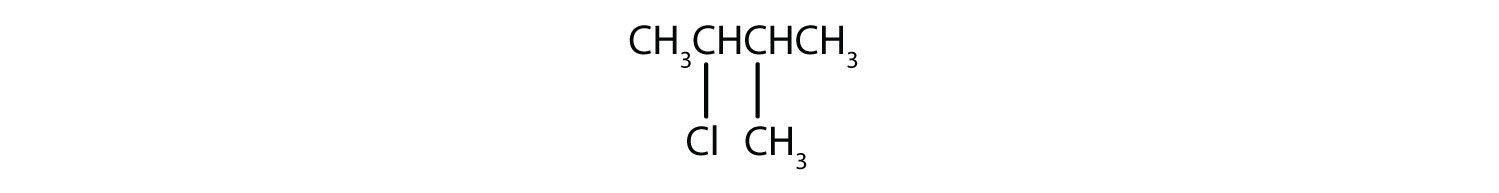
d)
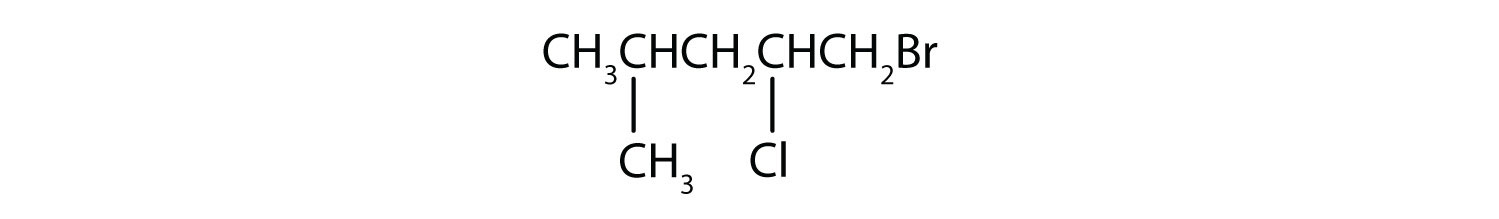
- Answer
-
1) a) The alkyl group (CH3CH2CH2–) is a propyl group, and the halogen is bromine (Br). The common name is therefore propyl bromide. For the IUPAC name, the prefix for bromine (bromo) is combined with the name for a three-carbon chain (propane), preceded by a number identifying the carbon atom to which the Br atom is attached, so the IUPAC name is 1-bromopropane.
b) The alkyl group [(CH3)2CH–] has three carbon atoms, with a chlorine (Cl) atom attached to the middle carbon atom. The alkyl group is therefore isopropyl, and the common name of the compound is isopropyl chloride. For the IUPAC name, the Cl atom (prefix chloro-) attached to the middle (second) carbon atom of a propane chain results in 2-chloropropane.
c) The alkyl group (CH3CH2–) is a ethyl group, and the halogen is iodine (I). The common name is therefore ethyl iodide. For the IUPAC name, the prefix for iodine (iodo) is combined with the name for a two-carbon chain (ethane), preceded by a number identifying the carbon atom to which the I atom is attached, so the IUPAC name is 1-iodoethane.
d) The alkyl group (CH3CH2CH2CH2–) is a butyl group, and the halogen is fluorine (F). The common name is therefore butyl fluoride. For the IUPAC name, the prefix for fluorine (Fluoro) is combined with the name for a four-carbon chain (butane), preceded by a number identifying the carbon atom to which the F atom is attached, so the IUPAC name is 1-fluorobutane.
2) a) The parent alkane has five carbon atoms in the longest continuous chain; it is pentane. A bromo (Br) group is attached to the second carbon atom of the chain. The IUPAC name is 2-bromopentane.
b) The parent alkane is hexane. Methyl (CH3) and bromo (Br) groups are attached to the second and fourth carbon atoms, respectively. Listing the substituents in alphabetical order gives the name 4-bromo-2-methylhexane.
c) 2-Chloro-3-methylbutane
d) 1-Bromo-2-chloro-4-methylpentane.
There is a fairly large distinction between the structural and physical properties of haloalkanes and the structural and physical properties of alkanes. As mentioned above, the structural differences are due to the replacement of one or more hydrogens with a halogen atom. The differences in physical properties are a result of factors such as electronegativity, bond length, bond strength, and molecular size.
Halogens and the Character of the Carbon-Halogen Bond
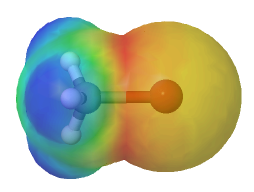
As discussed in Section 6.4, halogens are more electronegative than carbon. This results in a carbon-halogen bond that is polarized with the carbon atom bearing a partial positive charge and the halogen a partial negative charge. This polarity can be distinctly seen when viewing the electrostatic potential map of a methyl halide. Electron density is shown by a red/yellow color which is almost exclusively around the halogen atom. The methyl portion of the compound lacks electron density which is shown by a blue/green color.
The following image shows the relationship between the halogens and electronegativity. Notice, as we move up the periodic table from iodine to fluorine, electronegativity increases.
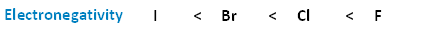
The following image shows the relationships between bond length, bond strength, and molecular size. As we progress down the periodic table from fluorine to iodine, molecular size increases. As a result, we also see an increase in bond length. Conversely, as molecular size increases, the bonds get longer, and the strength of those bonds decreases.

Haloalkanes Have Higher Boiling Points than Alkanes
When comparing alkanes and haloalkanes, we will see that haloalkanes have higher boiling points than alkanes containing the same number of carbons. London dispersion forces are the first of two types of forces that contribute to this physical property. You might recall from general chemistry that London dispersion forces increase with molecular surface area. In comparing haloalkanes with alkanes, haloalkanes exhibit an increase in surface area due to the substitution of a halogen for hydrogen. The increase in surface area leads to an increase in London dispersion forces, which then results in a higher boiling point.
Dipole-dipole interaction is the second type of force that contributes to a higher boiling point. As you may recall, this type of interaction is a coulombic attraction between the partial positive and partial negative charges that exist between carbon-halogen bonds on separate haloalkane molecules. Similar to London dispersion forces, dipole-dipole interactions establish a higher boiling point for haloalkanes in comparison to alkanes with the same number of carbons.

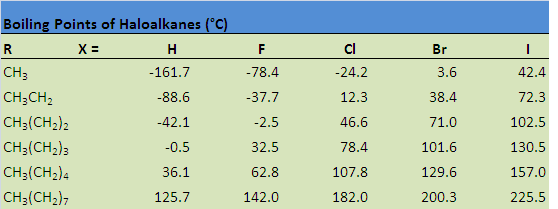
The table below illustrates how boiling points are affected by some of these properties. Notice that the boiling point increases when hydrogen is replaced by a halogen, a consequence of the increase in molecular size, as well as an increase in both London dispersion forces and dipole-dipole attractions. The boiling point also increases as a result of increasing the size of the halogen, as well as increasing the size of the carbon chain.
Solubility
Solubility in water
The alkyl halides are at best only slightly soluble in water. For a haloalkane to dissolve in water the attractions between the haloalkane molecules (van der Waals dispersion and dipole-dipole interactions) and hydrogen bonds between water molecules must be broken. Both of these cost energy. Energy is released when new intermolecular forces are generated between the haloalkane molecules and water molecules. These will only be dispersion forces and dipole-dipole interactions. These are not as strong as the original hydrogen bonds in the water, and so not as much energy is released as was used to separate the water molecules. The energetics of the changes are sufficiently "unprofitable" such that very little dissolves.
Solubility in organic solvents
Alkyl halides tend to dissolve in organic solvents because the new intermolecular attractions have roughly the same strength as the ones being broken in the separate haloalkane and solvent.
Chemical Reactivity
The pattern in chemical reactiviy lies in the strength of the bond between the carbon atom and the halogen atom. Previously in this section, it was noted that the trend for bond strength increases from C-I to C-Br to C-Cl with C-F bonds being the strongest. To react with the alkyl halides, the carbon-halogen bond has got to be broken. Because that gets easier as you go from fluoride to chloride to bromide to iodide, the compounds get more reactive in that order. Iodoalkanes are the most reactive and fluoroalkanes are the least. In fact, fluoroalkanes are so unreactive that we will ignore them completely from now on in this section!
Exercise \(\PageIndex{2}\)
1) Give the names of the following organohalides:
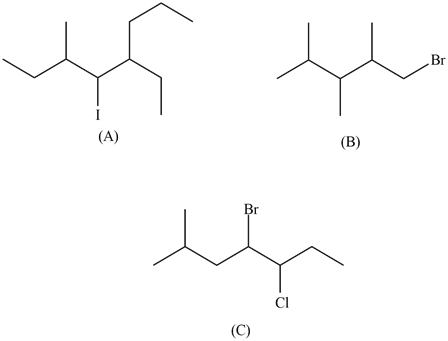
2) Draw the structures of the following compounds:
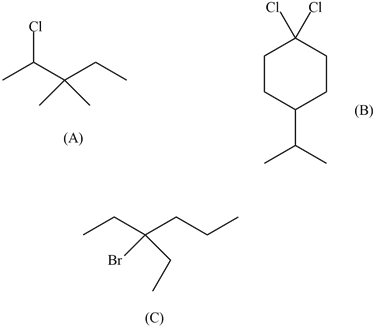
a) 2-Chloro-3,3-dimethylpentane
b) 1,1-Dichloro-4-isopropylcyclohexane
c) 3-bromo-3-ethylhexane
- Answer
-
1)
a) 5-ethyl-4-iodo-3-methyloctane
b) 1-bromo-2,3,4-trimethylpentane
c) 4-bromo-5-chloro-2-methylheptane
2)