12.1: Properties of Alcohols and Phenols
- Page ID
- 482358
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\(\newcommand{\ket}[1]{\left| #1 \right>} \)
\( \newcommand{\bra}[1]{\left< #1 \right|} \)
\( \newcommand{\braket}[2]{\left< #1 \vphantom{#2} \right| \left. #2 \vphantom{#1} \right>} \)
\( \newcommand{\qmvec}[1]{\mathbf{\vec{#1}}} \)
\( \newcommand{\op}[1]{\hat{\mathbf{#1}}}\)
\( \newcommand{\expect}[1]{\langle #1 \rangle}\)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)After completing this section, you should be able to
- explain why the boiling points of alcohols and phenols are much higher than those of alkanes, ethers, etc., of similar molecular mass.
- discuss the factors that are believed to determine the acidity of alcohols and phenols.
- list a given series of alcohols or phenols in order of increasing or decreasing acidity.
- explain the difference in acidity between two given alcohols or phenols.
- explain why phenols are more acidic than alcohols.
- explain, in terms of inductive and resonance effects, why a given substituted phenol is more or less acidic than phenol itself.
- write equations for the reactions of given alcohols and phenols with strong bases, such as sodium hydride and sodium amide.
Alcohols and phenols have nearly the same geometry around the oxygen atom as water. The R–O–H bond angle has an approximately tetrahedral value (108.5° in methanol, for instance), and the oxygen atom is sp3-hybridized.
The presence of a highly electronegative oxygen confers a measure of polar character to alcohols. Much of the electron density of alcohol is drawn towards the oxygen, giving alcohols a relatively high dipole moment (1.7 D for Methanol).
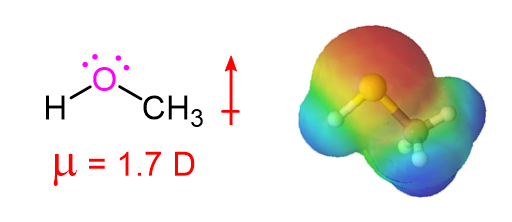
The Dipole Moment of Methanol
Boiling Points of Alcohols
Also, like water, alcohols, and phenols have higher boiling points than expected because of hydrogen bonding (Section 2.12). A positively polarized –OH hydrogen atom from one molecule is attracted to a lone pair of electrons on the electronegative oxygen atom of another molecule, resulting in a weak force that holds the molecules together (Figure \(\PageIndex{1}\)). These intermolecular attractions must be overcome for a molecule to break free from the liquid and enter the vapor state, so the boiling temperature is raised. For example, 1-propanol (MW = 60), butane (MW = 58), and chloroethane (MW = 65) have similar molecular weights, yet 1-propanol boils at 97 °C, compared with –0.5 °C for the alkane and 12.5 °C for the chloroalkane.
This table shows that alcohols (in blue) have higher boiling points than haloalkanes and alkanes with the equivalent molecular weight. It also shows that the boiling points of alcohols increase with the number of carbon atoms.
| Compound | IUPAC Name | Molecular Weight (g/mol) | Melting Point (oC) | Boiling Point (oC) |
|---|---|---|---|---|
| CH3OH | Methanol | 32.0 | -97.8 | 65.0 |
| CH3Cl | Chloromethane | 50.5 | -97.7 | -24.2 |
| CH3CH2OH | Ethanol | 46.1 | -114.7 | 78.5 |
| CH3CH2CH2CH3 | Butane | 58.1 | -140. | -1 |
| CH3CH2CH2OH | 1-Propanol | 60.1 | -126.5 | 97.4 |
| CH3CH2Cl | Chloroethane | 64.5 | -136.4 | 12.3 |
| CH3CH2CH2CH2CH3 | Pentane | 72.2 | -130 | 36.3 |
| CH3CH2CH2CH2OH | 1-Butanol | 74.1 | -89.5 | 117.3 |
| CH3(CH2)4OH | 1-Pentanol | 88.1 | -79 | 138 |
Solubility of Alcohols in Water
Alcohols and water have the ability to form hydrogen bonds with one another which tends to make the two liquids miscible. Small alcohols are completely soluble in water; mixing the two in any proportion generates a single solution. However, solubility decreases as the length of the hydrocarbon chain in the alcohol increases. At four carbon atoms and beyond, the decrease in solubility is noticeable; a two-layered substance may appear in a test tube when the two are mixed.
Acid-Base Properties of Alcohols
Another similarity with water is that alcohols and phenols are both weakly basic and weakly acidic. Alcohols are weak bases similar in strength to water and can accept protons from strong acids to form the conjugate acid called oxonium ions (ROH2+). An example is the reaction of methanol with hydrogen bromide to give methyloxonium bromide, which is analogous to the formation of hydroxonium bromide from the reaction of hydrogen bromide and water:
As weak bases, they are reversibly protonated by strong acids to yield oxonium ions, ROH2+.

Acidic behaviour of Alcohols
In aqueous solutions, alcohols dissociate slightly by donating a hydrogen to water. This creates the alcohol's conjugate base, called an alkoxide ion (RO-), along with hydronium (H3O+). The acid ionization constant (Ka) of ethanol is about 10-18, which is slightly less than that of water. Alcohols, such as ethanol, can be deprotonated to form its conjugate base by reaction with a stronger base, such as sodium amide (NaNH2), sodium hydride (NaH), or Grignard reagents (RMgBr). Alkoxides can also be formed using sodium or potassium metal which reacts vigorously but controllably with alcohols.
The strength of any acid HA in water can be expressed by an acidity constant, Ka.
\[K_{\mathrm{a}}=\frac{\left[\mathrm{A}^{-}\right]\left[\mathrm{H}_3 \mathrm{O}^{+}\right]}{[\mathrm{HA}]} \nonumber\]
with
\[\mathrm{p} K_{\mathrm{a}}=-\log K_{\mathrm{a}} \nonumber\]
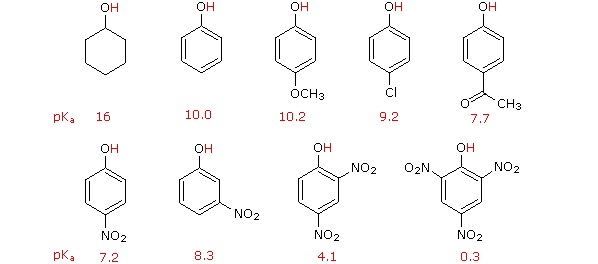
Compounds with a smaller Ka and larger pKa are less acidic, whereas compounds with a larger Ka and smaller pKa are more acidic. As shown in Table \(\PageIndex{1}\), simple alcohols like methanol and ethanol are about as acidic as water, but the more highly substituted tert-butyl alcohol is somewhat weaker. Substituent groups also have a significant effect: 2,2,2-trifluoroethanol is approximately 3700 times stronger than ethanol, for instance. Phenols and thiols, the sulfur analogs of alcohols, are substantially more acidic than water.
| Compound | pKa | |
|---|---|---|
| (CH3)3COH | 18 | |
| CH3CH2OH | 16 | |
| H2O | 15.74 | |
| CH3OH | 15.54 | |
| CF3CH2OH | 12.43 | |
| p-Aminophenol | 10.46 | |
| CH3SH | 10.3 | |
| p-Methylphenol | 10.17 | |
| Phenol | 9.89 | |
| p-Chlorophenol | 9.38 | |
| p-Nitrophenol | 7.15 |
The effect of alkyl substitution on alcohol acidity is due primarily to solvation of the alkoxide ion formed on acid dissociation. The more readily the alkoxide ion is solvated by water, the more stable it is, the more its formation is energetically favored, and the greater the acidity of the parent alcohol. For example, the oxygen atom of an unhindered alkoxide ion, such as that from methanol, is sterically accessible and is easily solvated by water. The oxygen atom of a hindered alkoxide ion, however, such as that from tert-butyl alcohol, is less easily solvated and is therefore less stable.
Inductive effects (Section 16.4) are also important in determining alcohol acidities. Electron-withdrawing halogen substituents, for instance, stabilize an alkoxide ion by spreading the charge over a larger volume, thus making the alcohol more acidic. Compare, for instance, the acidities of ethanol (pKa = 16) and 2,2,2-trifluoroethanol (pKa = 12.43), or of tert-butyl alcohol (pKa = 18) and nonafluoro-tert-butyl alcohol (pKa = 5.4).
Acidity of Alcohols in aqueous solution
In general, alcohols in aqueous solution are slightly less acidic than water. The order of acidity of various liquid alcohols generally is water > primary > secondary > tertiary ROH. By this we mean that the pKa is reduced as R is changed from primary to secondary to tertiary; therefore, tert-butyl alcohol is less acidic than ethanol. This trend is explained by the importance of solvation in equilibrium. In solution, the larger alkoxide ions, are less well solvated than the smaller ions, because fewer solvent molecules can be accommodated around the negatively charged oxygen in the larger ions. Acidity of alcohols therefore decreases as the size of the conjugate base increases. This trend can be clearly seen when comparing alkoxide size to the pKa of the corresponding alcohol listed in Table \(\PageIndex{2}\).
\[ ROH + OH^- \rightleftharpoons RO^- + HOH \nonumber \]
| R | Name | pKa1 |
|---|---|---|
| H | water | 14.0 |
| CH3 | methanol | 15.5 |
| CH3CH2 | ethanol | 15.9 |
| (CH3)2CH | propan-2-ol (isopropyl alcohol) | 16.5 |
| (CH3)3C | 2-methylpropan-2-ol (tert-butanol) | 17 |
| C6H5 (phenyl) | phenol | 9.95 |
The addition of an electron-withdrawing group, such as an electronegative halogen, can increase the acid strength of an alcohol by stabilizing its alkoxide conjugate base through induction (Section 2.10). The electron-withdrawing group helps to spread out the electron density of the alkoxide's negative charge, which has a stabilizing effect. The inductive effect is cumulative such that the acid strength of an alcohol becomes stronger (Lower pKa) as the number of halogens increases. The presence of nine fluorines in nonafluoro-tert-butyl alcohol decreases its pKa to 5.4 which is significantly more acidic than tert-butyl alcohol (pKa = 18). The electron-withdrawing effect of the fluorines is clearly seen when comparing the electrostatic potential maps of the corresponding alkoxides. In tert-butoxide the molecule's electron density is firmly centered around the oxygen as shown by the orange/yellow color. In nonafluoro-tert-butoxide the molecule's electron density is almost completely removed from the oxygen and shifted to the fluorines.

Reaction of Alcohols with Strong Bases
Because alcohols are weak acids, they don’t react with weak bases, such as amines or bicarbonate ion, and they only react to a limited extent with metal hydroxides such as NaOH. Alcohols do, however, react with alkali metals and with strong bases such as sodium hydride (NaH), sodium amide (NaNH2), and Grignard reagents (RMgX). Alkoxides are themselves bases that are frequently used as reagents in organic chemistry. They are named systematically by adding the -ate suffix to the name of the alcohol. Methanol becomes methanolate, for instance.
Acidity of Phenol
Phenols are about a million times more acidic than alcohols (Table \(\PageIndex{1}\)). The reason of this increasing acidity is the stability of the conjugate base through resonance effects. An excellent example of this effect is shown through phenol being roughly a million times more acidic than cyclohexanol.

Phenol is acidic enough to be deprotonated by weaker bases, such as sodium hydroxide (NaOH), to form the phenoxide ion. They are therefore soluble in dilute aqueous NaOH and can often be separated from a mixture simply by basic extraction into aqueous solution, followed by re-acidification.
Phenols are more acidic than alcohols because the phenoxide anion is resonance-stabilized. The increased acidity of phenol is caused by the negative charge and a set of lone pair electrons from the phenoxide's oxygen atom being delocalized by resonance to three different carbons on the aromatic ring. As a result, the negative charge is no longer entirely localized on the oxygen, but is spread throughout the whole ion allowing it to be highly stabilized.

Delocalization of the negative charge over the ortho and para positions of the aromatic ring results in increased stability of the phenoxide anion relative to undissociated phenol and in a consequently lower ∆G° for dissociation. Figure \(\PageIndex{2}\) compares electrostatic potential maps of an alkoxide ion (CH3O–) with phenoxide ion to show how the negative charge in phenoxide ion is delocalized from oxygen to the ring.
Acidity of Substituted Phenols
Substituted phenols can be either more acidic or less acidic than phenol itself, depending on whether the substituent is electron-withdrawing or electron-donating. Phenols with an electron-donating substituent are less acidic because these substituents concentrate the charge.
Phenols with an electron-withdrawing substituent, such as a nitro or carbonyl on the aromatic ring, are more acidic because these substituents delocalize the negative charge. For the conjugate base of the phenol derivative below, an additional resonance contributor can be drawn in which the negative formal charge is placed on the carbonyl oxygen.

Now the negative charge on the conjugate base can be spread out over two oxygens (in addition to three aromatic carbons). The phenol acid therefore has a pKa similar to that of a carboxylic acid, where the negative charge on the conjugate base is also delocalized to two oxygen atoms. The ketone group on the aromatic ring is acting as an electron withdrawing group and 'pulling' electron density towards itself, through both inductive and resonance effects.
The acidifying effect of an electron-withdrawing substituent is particularly noticeable in phenols with a nitro group at the ortho or para position.
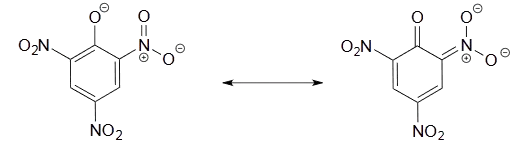
It is noteworthy that the influence of a nitro substituent is over ten times stronger in the para-location than it is meta, despite the fact that the latter position is closer to the hydroxyl group. This occurs since nitro groups at the meta position cannot accept the negative charge through resonance. Furthermore additional nitro groups have an additive influence if they are positioned ortho or para locations to the hydroxide. The trinitro compound shown at the lower right is a very strong acid called picric acid.
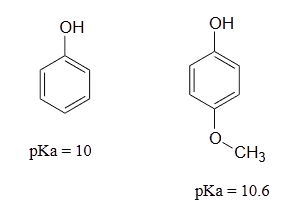
Lastly, if an electron donating group is attached to aromatic ring, as in p-methoxyphenol, the phenoxide ion is destabilized which causes a decrease in acidity in the corresponding phenol.
Is p-hydroxybenzaldehyde more acidic or less acidic than phenol?
Strategy
Identify the substituent on the aromatic ring, and decide whether it is electron-donating or electron-withdrawing. Electron-withdrawing substituents make the phenol more acidic by stabilizing the phenoxide anion, and electron-donating substituents make the phenol less acidic by destabilizing the anion.
Solution
We saw in Section 16.4 that a carbonyl group is electron-withdrawing. Thus, p-hydroxybenzaldehyde is more acidic (pKa = 7.9) than phenol (pKa = 9.89).

Using resonance structures, please explain why 4-methoxyphenol is less acidic than phenol.
- Answer
-
The methoxy group is an electron-donating group by resonance. A resonance contributor can be drawn in which a formal negative charge is placed on the carbon adjacent to the negatively-charged phenolate oxygen.

Because of like-charge repulsion, this destabilizes the negative charge on the phenolate oxygen, making the corresponding phenol less acidic.
The following data for isomeric four-carbon alcohols show that there is a decrease in boiling point with increasing substitution of the OH-bearing carbon. How might you account for this trend?
- 1-Butanol, bp 117.5 °C
- 2-Butanol, bp 99.5 °C
- 2-Methyl-2-propanol, bp 82.2 °C
- Answer
-
Hydrogen-bonding is more difficult in hindered alcohols.
Predict which compound of each pair is more soluble in water and explain your reasoning.
- Butan-1-ol or pentan-1-ol
- Phenol or cyclohexanol
- Octan-1,3-diol or octan-1-ol
- 1-Chlorohexane or hexan-1-ol
- Answer
-
- Butan-1-ol is more soluble in water because it has a smaller hydrophobic region compared to pentan-1-ol, allowing butan-1-ol to interact with water better.
- Phenol is more soluble in water than cyclohexanol because of the more polar character of its ring. phenol is able to interact with water better than cyclohexanol due to the conjugated pi-system of electrons in its ring, which which gives it a more ionic character.
- Octan-1,3-diol is more soluble in water as it has two hydroxy groups, allowing it to form more hydrogen bonds and interact with water better than octan-1-ol.
- Hexan-1-ol is more soluble in water as it can hydrogen bond compared to alkyl halides, such as 1-chlorohexane, which are insoluble in water.
Predict which compound has the higher boiling point and explain your reasoning.
- Water or ethanol
- Butan-1-ol or octan-1-ol
- Hexan-2-ol or hexan-2-one
- Answer
-
- Water has a higher boiling point compared to ethanol as it participates in more hydrogen bonding with other water molecules, thus requiring more energy to break the intermolecular attractions between water molecules.
- Octan-1-ol has the higher boiling point compared to butan-1-ol. Both alcohols can H-bond, however the longer hydrophobic carbon chain tail of octan-1-ol experiences more van der Waal interactions compared to the shorter hydrophobic region of butan-1-ol leading to a higher boiling point.
- Since hexan-1-ol can H-bond, it has a higher boiling point than hexan-2-one, which cannot H-bond.
The position of the electron-withdrawing substituent relative to the phenol hydroxyl is very important in terms of its effect on acidity. Which of the two substituted phenols below is more acidic? Use resonance drawings to explain your answer.

- Answer
-
The para-subsituted phenol is more acidic, because the negative charge on the conjugate base can be delocalized to the aldehyde oxygen. This is not possible when the aldehyde group is in the meta position

Rank the four compounds below from most acidic to least.
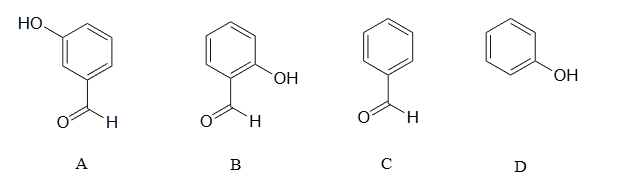
- Answer
-
Compound B is the strongest acid due to electron-withdrawing resonance effects-the negative charge on the conjugate base can be delocalized to the aldehyde oxygen. Compound A is the 2nd strongest acid -while the negative charge on the conjugate base cannot be delocalized to the aldehyde oxygen due to the meta-position, the aldehyde none the less has a stabilizing, electron-withdrawing inductive effect. Compound D is ranked #3 -it is phenol, and does not have any electron-withdrawing substituents on the ring as do B and A. Compound C is the least acidic -neither the phenyl group nor the aldehyde are even slightly acidic.
Rank the following substances in order of increasing acidity:
- \(\ce{(CH3)2CHOH}\), \(\ce{HC≡CH}\), \(\ce{(CF3)2CHOH}\), \(\ce{CH3OH}\)
- Phenol, p-methylphenol, p-(trifluoromethyl)phenol
- Benzyl alcohol, phenol, p-hydroxybenzoic acid
- Answer
-
- \(\ce{HC≡CH < (CH3)2CHOH < CH3OH < (CF3)2CHOH}\)
- p-Methylphenol < Phenol < p-(Trifluoromethyl)phenol
- Benzyl alcohol < Phenol < p-Hydroxybenzoic acid
p-Nitrobenzyl alcohol is more acidic than benzyl alcohol, but p-methoxybenzyl alcohol is less acidic. Explain.
- Answer
-
The electron-withdrawing nitro group stabilizes an alkoxide ion, but the electron-donating methoxyl group destabilizes the anion.
Use a resonance argument to explain why picric acid has such a low pKa.
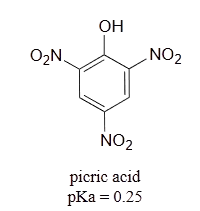
- Answer
-
The negative charge on the conjugate base of picric acid can be delocalized to oxygen atoms on all threeof the nitro groups. One such resonance contributor is shown below. This extensive delocalization means that the conjugate base is very stable, and the conjugate acid is thus a very strong acid.