10.8: Organometallic Coupling Reactions
- Page ID
- 482379
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Many other kinds of organometallic compounds can be prepared in a manner similar to that of Grignard reagents. For instance, alkyllithium reagents, RLi, can be prepared by the reaction of an alkyl halide with lithium metal. Alkyllithiums are both nucleophiles and strong bases, and their chemistry is similar in many respects to that of alkylmagnesium halides.
Gilman Reagents
One particularly valuable reaction of alkyllithiums occurs when making lithium diorganocopper compounds, R2CuLi, by reaction with copper(I) iodide in diethyl ether as solvent. Often called Gilman reagents (LiR2Cu), lithium diorganocopper compounds are useful because they undergo a coupling reaction with organochlorides, bromides, and iodides (but not fluorides). One of the alkyl groups from the lithium diorganocopper reagent replaces the halogen of the organohalide, forming a new carbon–carbon bond and yielding a hydrocarbon product. Lithium dimethylcopper, for instance, reacts with 1-iododecane to give undecane in a 90% yield.
Example
This organometallic coupling reaction is useful in organic synthesis because it forms carbon–carbon bonds, thereby allowing the preparation of larger molecules from smaller ones. As the following examples indicate, the coupling reaction can be carried out on aryl and vinylic halides as well as on alkyl halides.
An organocopper coupling reaction is carried out commercially to synthesize muscalure, (9Z)-tricosene, the sex attractant secreted by the common housefly. Minute amounts of muscalure greatly increase the lure of insecticide-treated fly bait and provide an effective and species-specific means of insect control.
Mechanism
The mechanism of the coupling reaction involves initial formation of a triorganocopper intermediate, followed by coupling and loss of a mono-organocopper, RCu. The coupling is not a typical polar nucleophilic substitution reaction of the sort considered in the next chapter.
The Suzuki-Miyaura Reaction
In addition to the coupling reaction of diorganocopper reagents with organohalides, related processes also occur with other organometallic reagents, particularly organopalladium compounds. One of the most commonly used procedures is the coupling reaction of an aromatic or vinyl substituted boronic acid [R—B(OH)2] with an aromatic or vinyl substituted organohalide in the presence of a base and a palladium catalyst. This reaction is less general than the diorganocopper reaction because it doesn’t work with alkyl substrates, but it is preferred when possible because it uses only a catalytic amount of metal rather than a full equivalent and because palladium compounds are less toxic than copper compounds. For example:
Called the Suzuki–Miyaura reaction, this process is particularly useful for preparing so-called biaryl compounds, which have two linked aromatic rings. A large number of commonly used drugs fit this description, so the Suzuki–Miyaura reaction is much-used in the pharmaceutical industry.
Example
As an example, valsartan, marketed as Diovan, is widely prescribed to treat high blood pressure, heart failure, and diabetic kidney disease. Its synthesis begins with a Suzuki–Miyaura coupling of ortho-chlorobenzonitrile with para-methylbenzeneboronic acid.
Mechanism
Shown in a simplified form in Figure , the mechanism of the Suzuki–Miyaura reaction involves initial reaction of the aromatic halide with the palladium catalyst to form an organopalladium intermediate, followed by reaction of that intermediate with the aromatic boronic acid. The resultant diorganopalladium complex then decomposes to the coupled biaryl product plus regenerated catalyst.
Starting with alkyl halides containing no more than four carbon atoms, how would you synthesize each of the following alkanes?
- 2,5-dimethylhexane
- 2-methylhexane
- Answer
- Notice that in (a), both the alkyl halides are primary. This fact should ensure a good yield of product.

- In (b) we have the choice of using 2-bromopropane and 1-bromobutane, or 1-bromo-2-methylpropane and 1-bromopropane. We chose the latter as it enables us to use two primary alkyl halides, and hence a simpler procedure.
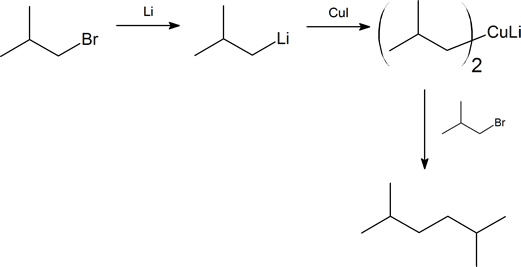
How would you carry out the following transformations using an organocopper coupling reaction? More than one step is required in each case.
- Answer
-
- 1. NBS; 2. (CH3)2CuLi
- 1. Li; 2. CuI; 3. CH3CH2CH2CH2Br
- 1. BH3; 2. H2O2, NaOH; 3. PBr3; 4. Li, then CuI; 5. CH3(CH2)4Br





