14.10: Synthesis of Ethers
- Page ID
- 134804
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Objectives
After completing this section, you should be able to
- write an equation to illustrate the industrial preparation of simple symmetrical ethers.
- write an equation to illustrate the Williamson synthesis of ethers.
- identify the ether obtained from the reaction of a given alkyl halide with a given alkoxide ion.
- identify the reagents needed to prepare a given ether through a Williamson synthesis.
- identify the limitations of the Williamson synthesis, and make the appropriate choices when deciding how best to synthesize a given ether.
- write an equation to describe the formation of an alkoxide from an alcohol.
- identify silver(I) oxide as a reagent which can be used in a Williamson synthesis.
- write an equation to show how an ether can be prepared by the alkoxymercuration‑demercuration of an alkene.
- identify the product formed from the alkoxymercuration‑ demercuration of a given alkene.
- identify the alkene, the reagents, or both, needed to prepare a given ether by the alkoxymercuration‑demercuration process.
- write the detailed mechanism of the reaction between an alkene, an alcohol and mercury(II) trifluoroacetate.
Key Terms
Make certain that you can define, and use in context, the key terms below.
- alkoxymercuration
- oxymercuration
- Williamson ether synthesis
Study Notes
We studied oxymercuration as a method of converting an alkene to an alcohol in Section 8.4. “Alkoxymercuration” is a very similar process, except that we are now converting an alkene into an ether. The two processes are compared below.
| In | oxymercuration | alkoxymercuration |
|---|---|---|
| we react an | alkene | alkene |
| with | water | an alcohol |
| in the presence of | Hg(O2CCH3)2 | Hg(O2CCF3)2 |
| followed by treatment with | NaBH4 | NaBH4 |
| to produce an | alcohol | ether |
Review the mechanism of the oxymercuration reaction in Section 8.4, paying particular attention to the regiochemistry and the stereochemistry of the reaction. The mechanism is identical to alkoxymercuration.
Ethers are usually prepared from alcohols or their conjugate bases. One important procedure, known as the Williamson Ether Synthesis, proceeds by an SN2 reaction of an alkoxide nucleophile with an alkyl halide. Reactions #1 and #2 below are two examples of this procedure. When applied to an unsymmetrical ether, as in this case, there are two different combinations of reactants are possible. Of these one is usually better than the other. Since alkoxide anions are strong bases, the possibility of a competing E2 elimination must always be considered. Bearing in mind the factors that favor substitution over elimination, a 1º-alkyl halide should be selected as a preferred reactant whenever possible. Thus, reaction #1 gives a better and cleaner yield of benzyl isopropyl ether than does reaction #2, which generates considerable elimination product.
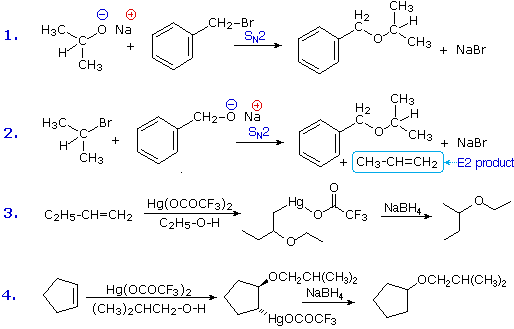
A second general ether synthesis, alkoxymercuration, is patterned after the oxymercuration reaction. Reactions #3 and #4 are examples of this two-step procedure. Note that the alcohol reactant is used as the solvent, and a trifluoroacetate mercury (II) salt is used in preference to the acetate (trifluoroacetate anion is a poorer nucleophile than acetate). The mechanism of alkoxymercuration is similar to that of oxymercuration, with an initial anti-addition of the mercuric species and alcohol being followed by reductive demercuration.
Acid-catalyzed dehydration of small 1º-alcohols constitutes a specialized industrial method of preparing symmetrical ethers. As shown in the following two equations, the success of this procedure depends on the temperature. At 110º to 130 ºC an SN2 reaction of the alcohol conjugate acid leads to an ether product.
\[\ce{2 CH_3CH_2-OH + H_2SO_4 ->[130\;^oC] CH_3CH_2\bond{-}O\bond{-}CH_2CH_3 + H_2O} \tag{18.2.1}\]
At higher temperatures (over 150 ºC) an E2 elimination takes place.
\[\ce{CH3CH_2-OH + H_2SO_4 ->[150\;^oC] CH_2\bond{=}CH_2 + H_2O} \tag{18.2.2}\]
This reaction cannot be employed to prepare unsymmetrical ethers. It is because a mixture of products is likely to be obtained.
Exercises
Contributors
Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)
Prof. Steven Farmer (Sonoma State University)
William Reusch, Professor Emeritus (Michigan State U.), Virtual Textbook of Organic Chemistry

