5.5: Oxidation-Reduction (Redox) Reactions
- Page ID
- 165671
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Skills to Develop
- Define oxidation and reduction in terms of a gain or loss of oxygen or hydrogen atoms.
- Identify the substances involved in oxidation and reduction in a reaction.
- Identify the oxidizing and reducing agents in a redox reaction.
- To identify a chemical reaction as an oxidation-reduction reaction.
Oxygen's Role in Reactions
Oxygen is an element that has been known for centuries. In its pure elemental form, oxygen is highly reactive, and it readily makes compounds with most other elements. It is also the most abundant element by mass in the Earth's crust. The class of reactions called oxidation and reduction were originally defined with respect to the element oxygen.
Early scientists viewed oxidation as a process in which a substance combines with oxygen to produce oxides. Examples of oxidation are shown below: magnesium, methane, iron and copper are being oxidized.
The heating of magnesium in air allows it to combine with oxygen from the air to form magnesium oxide.
\[2 \ce{Mg} \left( s \right) + \ce{O_2} \left( g \right) \rightarrow 2 \ce{MgO} \left( s \right)\]
The burning (combustion) of methane allows the fuel (methane) to react with oxygen (from the air), producing carbon dioxide and water (and heat).
\[\ce{CH_4} \left( g \right) + 2 \ce{O_2} \left( g \right) \rightarrow \ce{CO_2} \left( g \right) + 2 \ce{H_2O} \left( g \right)\]
The oxidation (commonly known as rusting or corrosion) of iron is shown below. Iron that is exposed to air (and water) is slowly oxidized to produce rust (an oxide of iron).
\[4 \ce{Fe} \left( s \right) + 3\ce{O_2} \left( g \right) \rightarrow 2 \ce{Fe_2O_3} \left( s \right)\]
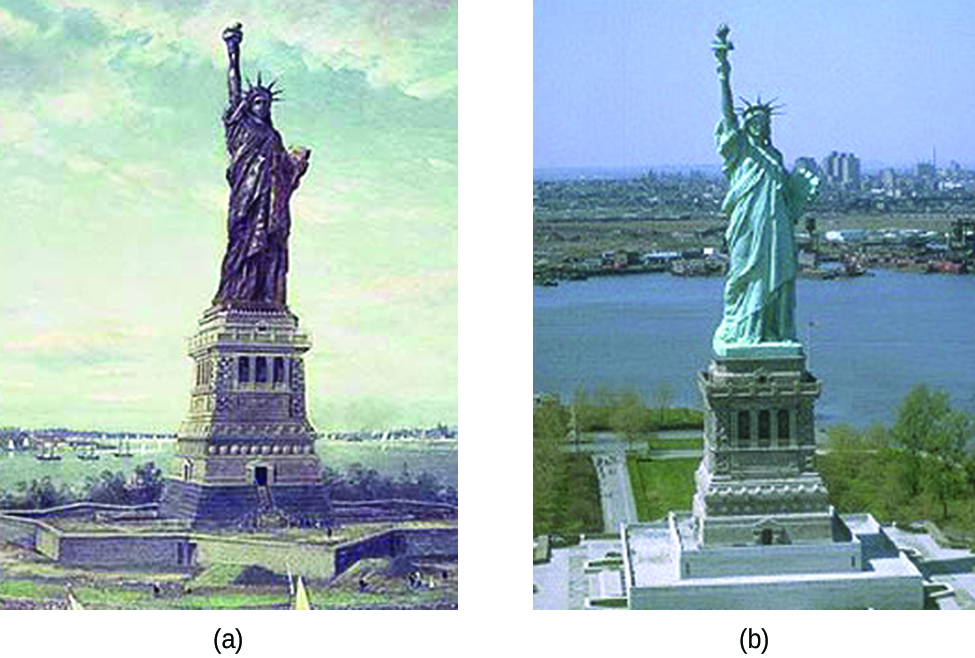
The Statue of Liberty is a landmark every American recognizes. The Statue of Liberty is easily identified by its height, stance, and unique blue-green color. When this statue was first delivered from France, its appearance was not green. It was brown, the color of its copper “skin.” So how did the Statue of Liberty change colors? The change in appearance was a direct result of corrosion. The copper that is the primary component of the statue slowly underwent oxidation from the air. The oxidation-reduction reactions of copper metal in the environment occur in several steps. Copper metal is oxidized to copper(I) oxide Cu2O), which is red, and then to copper(II) oxide (CuO), which is black.
\[4 \ce{Cu} \left( s \right) + \ce{O_2} \left( g \right) \rightarrow 2 \ce{Cu_2O} \left( s \right)\]
\[2 \ce{Cu_2O} \left( s \right) + \ce{O_2} \left( g \right) \rightarrow 4 \ce{CuO} \left( s \right)\]
Coal, which was often high in sulfur, was burned extensively in the early part of the last century. As a result, sulfur trioxide, carbon dioxide, and water all reacted with the CuO. These three compounds are responsible for the characteristic blue-green patina seen today. Fortunately, formation of the patina created a protective layer on the surface, preventing further corrosion of the copper skin.
Bleaches contain various compounds such as sodium hypochlorite (NaClO) that can oxidize stains by the transfer of oxygen atoms, making the molecules in the stains more water-soluble and therefore easier to rinse off. Hydrogen peroxide (H2O2) releases oxygen as it spontaneously decomposes. It also acts as a bleach and is used as an antiseptic that kills bacteria by oxidizing them.
We worry a lot about our smiles. Over the years teeth do discolor some, so the procedure of teeth bleaching has become more and more popular. Best done in a dentist's office, various chemical preparations containing peroxides are used to whiten teeth. Less effective, but easier to use are "teeth-whitening" toothpastes (also containing peroxides) that promise to give you a brighter smile and improve your life in every way.
Oxidation and Reduction
The opposite of oxidation is called reduction. Since oxidation was originally defined as the addition of oxygen, reduction was therefore the removal of oxygen from a substance. Many naturally occurring metal ores are present as oxides. The pure metals can be extracted by reduction. For example, pure iron is obtained from iron (III) oxide by reacting it with carbon at high temperatures.
\[2 \ce{Fe_2O_3} \left( s \right) + 3 \ce{C} \left( s \right) \rightarrow 4 \ce{Fe} \left( s \right) + 3 \ce{CO_2} \left( g \right)\]
The removal of oxygen from the Fe2O3 molecule means that it is being reduced to Fe. Note that an oxidation process is also occurring simultaneously in this reaction; the carbon reactant is being oxidized to CO2. This is an important concept. In looking at organic compounds, we can describe oxidation and reduction in terms of the loss or gain of oxygen or the loss or gain of hydrogen. The changes in number of oxygen atoms, and number of hydrogen atoms is summarized below for oxidation and reduction reactions.
| Process | Change in oxygen (some reactions) | Change in hydrogen (some reactions) |
|---|---|---|
| Oxidation | gain | lose |
| Reduction | lose | gain |
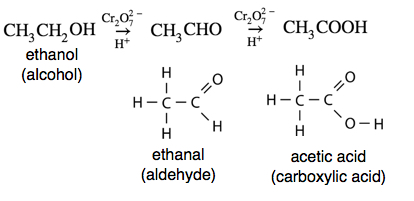
If we look at the oxidation of ethanol (CH3CH2OH, an alcohol) to form ethanal (CH3CHO, an aldehyde), we see that the number of bonds to oxygen has increased and the number of hydrogen atoms has decreased from six to four. Either or both of these indicate that an oxidation has occurred.

For a reduction, we look for the removal of oxygen or bonds to oxygen or the addition or hydrogen atoms. In the following reaction, we see ethanoic acid (CH3COOH, a carboxylic acid) being reduced to ethanal (CH3CHO, an aldehyde). Note that the number of oxygen atoms is reduced from two to one. There is no change in the number of hydrogen atoms, but both changes do not need to happen for evidence of oxidation or reduction.

If we see evidence of an oxidation or reduction, we know the other must have happened as well. Oxidation and reduction must happen together. Neither can happen alone in a reaction.
Oxidation and Reduction Must Occur at the same time
Note that an oxidation process is simultaneously occurring with a reduction process. Oxidation cannot happen alone, without reduction. If reactant A is gaining oxygen (getting oxidized), reactant B must be losing the O (getting reduced). If reactant A is undergoing the removal of hydrogen (getting oxidized), reactant B must be undergoing the addition of hydrogen (getting reduced).
Redox reactions are also commonly described in terms of oxidizing and reducing agents. In the reaction below, C is being oxidized by gaining oxygen. The source of oxygen is Fe2O3. In other words, Fe2O3 is causing the C to be oxidized. Consequently, Fe2O3 is referred to as the oxidizing agent. Conversely, the C causes the Fe2O3 to lose oxygen and become reduced, so C is the reducing agent. An oxidizing agent (OA) is a substance that causes oxidation by releasing oxygen, and a reducing agent (RA) is a substance that causes reduction by gaining oxygen. Said another way, the oxidizing agent (OA) is the substance that is reduced, while the reducing agent (RA) is the substance that is oxidized. Focusing on the reaction below, Fe2O3 is the substance being reduced, while C is the substance that is being oxidized. Hence, Fe2O3 is the oxidizing agent (OA) and C is the reducing agent (RA).
\[2 \ce{Fe_2O_3} \left( s \right) + 3 \ce{C} \left( s \right) \rightarrow 4 \ce{Fe} \left( s \right) + 3 \ce{CO_2} \left( g \right)\]
Fe2O3 = substance reduced C = substance oxidized
Fe2O3 = oxidizing agent (OA) C = reducing agent (RA)
Note
The substance oxidized and substance reduced are both reactants in the reaction.
Several classes of organic compounds are related to one another by oxidation and reduction reactions. Alkanes, alkenes, and alkynes represent different levels of oxidation of a hydrocarbon. When an alkane is heated in the presence of an appropriate catalyst, it can be oxidized to the corresponding alkene in a reaction called a dehydrogenation reaction. Two hydrogen atoms are removed in the process. The alkene can be further oxidized to an alkyne by the removal of two more hydrogen atoms.
oxidation: CH3CH3→CH2=CH2→CH≡CH
The reactions are reversible, so an alkyne can be reduced first to an alkene and then to an alkane. These are addition reactions.
reduction: CH≡CH→CH2=CH2→CH3CH3
The alkane is the most reduced form of a hydrocarbon, while the alkyne is the most oxidized form.
Oxidation reactions in organic chemistry often involve the addition of oxygen to a compound (or an increase in the number of bonds to oxygen), which changes the functional group that is present. The following sequence shows how methane can be oxidized first to methanol (alcohol), then to methanal (aldehyde), then to methanoic acid (carboxylic acid), and finally to carbon dioxide. Each step in the process is either a gain of oxygen or a loss of hydrogen. Each step also represents energy, which explains why the complete combustion of alkanes to carbon dioxide is an extremely exothermic reaction. Hence, alkanes are used as fuels.
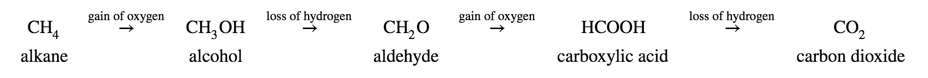
The opposite process is the reduction of a carboxylic acid to an alkane which involves the loss of oxygen and the gain of hydrogen.
When a primary alcohol is oxidized to an aldehyde in the presence of water, the reaction can be difficult to stop because the aldehyde can be further oxidized to the corresponding carboxylic acid. For example, the oxidation of ethanal produces ethanoic (acetic) acid. Ethanol-containing beverages such as wine are susceptible to such oxidation if kept for long periods of time after having been opened and exposed to the air. Wine that has become oxidized will have an unpleasant vinegary taste due to the production of acetic acid.
Summary
- Oxidation is the gain of oxygen (O) while reduction is the loss of oxygen (O).
- Oxidation is the loss of hydrogen (H) while reduction is the gain of hydrogen (H).
- Oxidation and reduction reactions must occur together.
- The reactant oxidized is the reducing agent.
- The reactant reduced is the oxidizing agent.
To Your Health: Redox Reactions and Pacemaker Batteries
All batteries use redox reactions to supply electricity because electricity is basically a stream of electrons being transferred from one substance to another. Pacemakers—surgically implanted devices for regulating a person’s heartbeat—are powered by tiny batteries, so the proper operation of a pacemaker depends on a redox reaction.
Pacemakers used to be powered by NiCad batteries, in which nickel and cadmium (hence the name of the battery) react with water according to this redox reaction:
Cd(s) + 2NiOOH(s) + 2H2O(ℓ) → Cd(OH)2(s) + 2Ni(OH)2(s)
The cadmium is oxidized, while the nickel atoms in NiOOH are reduced. Except for the water, all the substances in this reaction are solids, allowing NiCad batteries to be recharged hundreds of times before they stop operating. Unfortunately, NiCad batteries are fairly heavy batteries to be carrying around in a pacemaker. Today, the lighter lithium/iodine battery is used instead. The iodine is dissolved in a solid polymer support, and the overall redox reaction is as follows:
2Li(s) + I2(s) → 2LiI (s)
Lithium is oxidized, and iodine is reduced. Although the lithium/iodine battery cannot be recharged, one of its advantages is that it lasts up to 10 years. Thus, a person with a pacemaker does not have to worry about periodic recharging; about once per decade a person requires minor surgery to replace the pacemaker/battery unit. Lithium/iodine batteries are also used to power calculators and watches.

Example \(\PageIndex{1}\)
In each conversion, indicate whether oxidation or reduction is occurring.
- N2 → NH3
- CH3CH2OHCH3 → CH3COCH3
- HCHO → HCOOH
SOLUTION
- Hydrogen is being added to the original reactant molecule, so reduction is occurring.
- Hydrogen is being removed from the original reactant molecule, so oxidation is occurring.
- Oxygen is being added to the original reactant molecule, so oxidation is occurring.
Exercise \(\PageIndex{1}\)
In each conversion, indicate whether oxidation or reduction is occurring.
- CH4 → CO2 + H2O
- NO2 → N2
- CH2=CH2 → CH3CH3
- Answer
-
- oxidation
- reduction
- reduction
Summary
Chemical reactions in which oxygen and/or hydrogen are transferred are called oxidation-reduction, or redox, reactions. Oxidation is the gain of O or loss of H. Reduction is the loss of O or gain of H. Oxidation and reduction always occur together, even though they can be written as separate chemical equations.
Concept Review Exercises
-
Give two different definitions for oxidation and reduction.
-
Give an example of each definition of oxidation and reduction.
Answers
-
Oxidation is the addition of oxygen or the loss of hydrogen; reduction is the loss of oxygen or the addition of hydrogen.
-
C → CO2 (oxidation); C2H4 + H2 → C2H6 (reduction) (answers will vary)
Exercises
-
For the following redox reactions, identify the substance oxidized and the substance reduced.
- 2C2H2 + 5O2 →4CO2 + 2H2O
- 4Al + 3O2 → 2Al2O3
- H2O2 + H2 → 2H2O
- 2CH3OH + 3O2 → 2CO2 + 4H2O
2. Identify the oxidizing and reducing agent in each redox reaction below.
- 2C2H2 + 5O2 →4CO2 + 2H2O
- 4Al + 3O2 → 2Al2O3
- H2O2 + H2 → 2H2O
- 2CH3OH + 3O2 → 2CO2 + 4H2O
Answers
1. Look at the reactants, and examine which is the substance oxidized and the substance reduced.
- substance oxidized:C2H2 since it lost H (and gained O) substance reduced: O2 since it gained H
- substance oxidized:Al since Al gained O substance reduced: O2
- substance oxidized:H2 since it gained O substance reduced: H2O2 since it lost O
- substance oxidized:CH3OH since it lost H (and gained O) substance reduced: O2 since it gained H
2. Identify the oxidizing and reducing agent in each reaction below.
The substance oxidized is the RA while the substance reduced is the OA.
- RA: C2H2 OA: O2
- RA: Al OA: O2
- RA: H2 OA: H2O2
- RA: CH3OH OA: O2

