1.6: Sample exam questions and review
- Page ID
- 240458
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Below are the slides for this unit. The text in between is just to make them searchable. So if you find a word like "anion" in a search, the slide set above with contain that word. If you want print-outs, here is a PDF file with 6 slides per sheet (comes out to 8 double-sided pages of paper).
 Chemistry 0103
Chemistry 0103
Part 1: Atoms and Bonds (Chapters 1 – 3)
1.1 “Stuff” Is Matter
* “Stuff,” or matter, is anything that takes up space and has mass.
* Examples of matter: a book, a bottle, air in a balloon, or a chair.
Volume
* Volume is a quantity to measure how much space is take up by something
* In which units do we measure volume?
* How do we measure volume?
Matter: Mass
* Matter has mass and can be weighed.
* Mass remains the same regardless of location (it takes effort to launch a rocket on earth or in outer space)
* Mass and weight are related through gravity. On earth, measuring weight is convenient.
 Matter: Mass
Matter: Mass
* What units do we use to measure mass?
* How do we measure mass?
Mass vs. weight
* The weight of an object is determined its mass and by gravity.
* Gravity changes with location.
* Compare the weight of an astronaut on the moon and on Earth
* Compare the mass of an astronaut on the moon and on Earth
Measuring mass
* Scales, or balances, are used to measure mass.
* Scales are calibrated so that a known mass standard shows the expected result regardless of the gravitational pull of Earth.
Units of measurements
* Time = 3 hours
* Without a unit, the number “3” is meaningless
* Always indicate which quantity you are measuring
Metric system
* Length l: meter (m)
* Volume V: liter (L)
* Mass m: kilogram (kg)
* Time t: seconds (s)
* not metric: feet, gallons/barrels, pounds, semesters
Smaller (and bigger) units
* 1 km = 1000 m
* 1 ms = 0.001 s or 1000 ms = 1 m
* 1 µL = 0.000001 L or 1,000,000 µL = 1 L
 Converting units
Converting units
* Convert 100 mg to grams
* 1 mg = 0.001 g or 1000 mg = 1 g.
States of matter
* (s): solid, has definite shape and volume.
Atoms in a solid are tightly packed (touch their neighbors).
* (l): liquid, has a definite volume, but its shape changes depending on the container it is in.
Atoms in a liquid touch their neighbors, but can move large distances.
* (g): gas, has no definite volume or shape.
Atoms in a gas don’t touch their neighbors.
Classifying matter
Periodic Table
* Shows all the elements found on Earth.
* Online, e.g. http://www.ptable.com/
Periodic Table
* Letters represent the elemental symbol and represent the name of the element and the atom type, e.g. C: Carbon.
* Many of the elements have symbols derived directly from the name of the element. The first letter of the symbol is always capitalized, and if a second letter is present, it is lowercase. For example, H = hydrogen, Li = lithium.
* Some elements have symbols that do not match the first letters of their name. For example, sodium = Na, gold = Au. Their symbols are derived from the Latin names of the element.
Periodic Table: Groups
* A group is a vertical column of blocks. Each group has a number and letter designation. Groups 1A–8A are main group elements. Groups with B designations are transition elements.
* Group 1A are the alkali metals.
* Group 2A are the alkaline earth metals.
* Group 7A are the halogens.
* Group 8A are the noble gases.
 Periodic Table: Periods
Periodic Table: Periods
* A period is a horizontal row of blocks. The periods are numbered 1 to 7 with sections of periods 6 and 7 set apart at the bottom of the periodic table.
* Lanthanides are Period 6 elements with atomic numbers of 58–71, which are set apart at the bottom of the periodic table.
* Actinides are Period 7 elements with atomic numbers of 90–103, which are set apart at the bottom of the periodic table.
Physical vs. chemical change
* Physical vs. chemical change
* A physical change results in a change in the form of matter, but its identity remains unchanged.
* No changes in chemical bonds
* Water, H2O, can undergo a physical change from solid to liquid to gas.
* Chemical change: usually called chemical reaction
Chemical Reaction
* A chemical reaction results in a change in the chemical identity of the substance or substances involved.
* A chemical reaction occurs when a substance or substances undergoes a chemical change.
* When baking soda (sodium bicarbonate) is added to a cake recipe, it undergoes a chemical reaction, when heated, to produce carbon dioxide and water vapor plus sodium carbonate.
Chemical Equations
Chemical Equations Summary
* A chemical equation is the chemist’s way of summarizing what happens in a chemical reaction.
* Reactants left, products right
* The arrow can be read as “react to form.” Reaction conditions, like heat, are written above or below the arrow.
* The labels in parentheses after each substance indicate its physical state: (s)olid, (l)iquid, (g)as, or (aq)ueous.
Subatomic Particles
* An atom is the smallest part of an element that has the properties of the element.
* An atom is made up of three subatomic particles
 Structure of an Atom
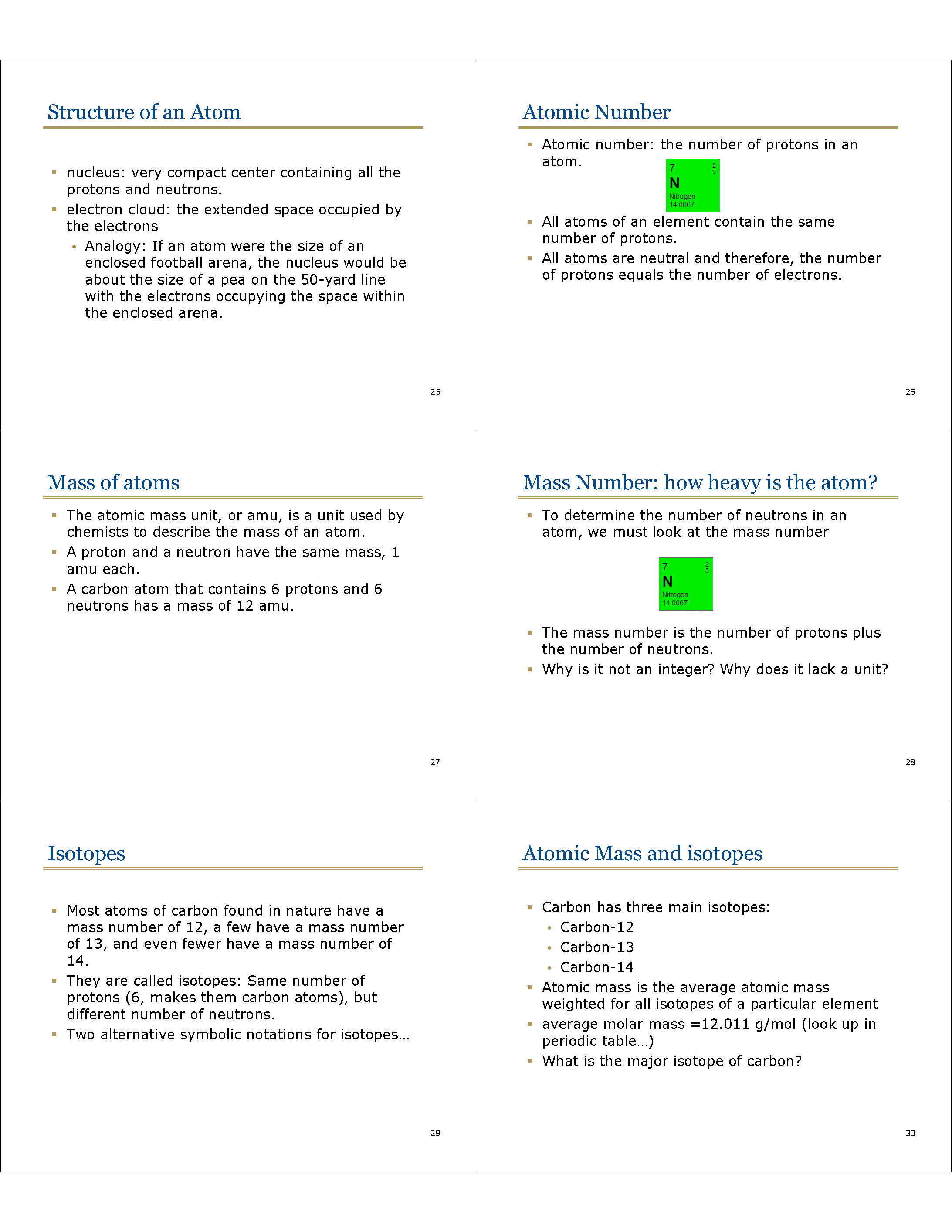
Structure of an Atom
* nucleus: very compact center containing all the protons and neutrons.
* electron cloud: the extended space occupied by the electrons
• Analogy: If an atom were the size of an enclosed football arena, the nucleus would be about the size of a pea on the 50-yard line with the electrons occupying the space within the enclosed arena.
Atomic Number
* Atomic number: the number of protons in an atom.
* All atoms of an element contain the same number of protons.
* All atoms are neutral and therefore, the number of protons equals the number of electrons.
Mass of atoms
* The atomic mass unit, or amu, is a unit used by chemists to describe the mass of an atom.
* A proton and a neutron have the same mass, 1 amu each.
* A carbon atom that contains 6 protons and 6 neutrons has a mass of 12 amu.
Mass Number: how heavy is the atom?
* To determine the number of neutrons in an atom, we must look at the mass number
* The mass number is the number of protons plus the number of neutrons.
* Why is it not an integer? Why does it lack a unit?
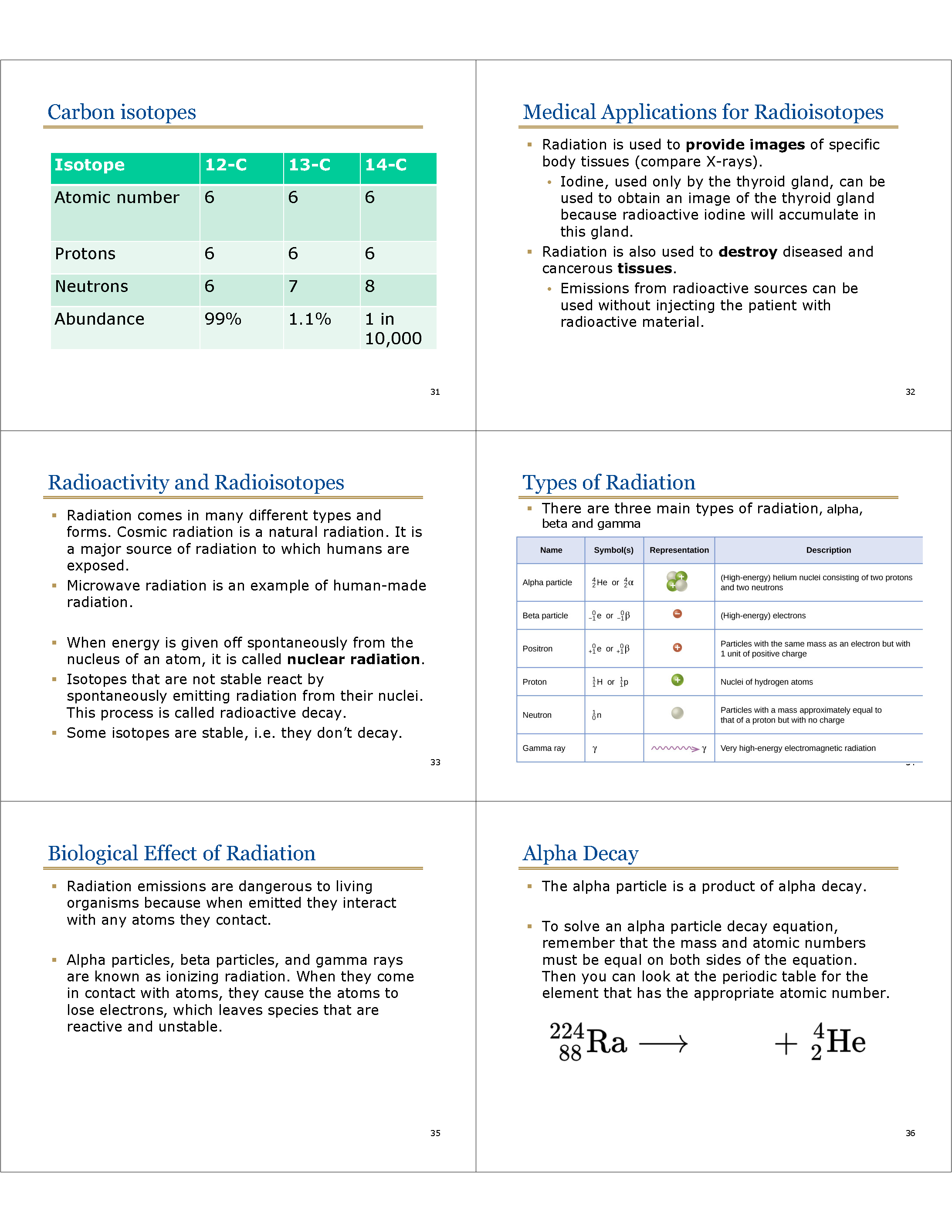
Isotopes
* Most atoms of carbon found in nature have a mass number of 12, a few have a mass number of 13, and even fewer have a mass number of 14.
* They are called isotopes: Same number of protons (6, makes them carbon atoms), but different number of neutrons.
* Two alternative symbolic notations for isotopes…
Atomic Mass and isotopes
* Carbon has three main isotopes:
• Carbon-12
• Carbon-13
• Carbon-14
* Atomic mass is the average atomic mass weighted for all isotopes of a particular element
* average molar mass =12.011 g/mol (look up in periodic table…)
* What is the major isotope of carbon?
 Carbon isotopes
Carbon isotopes
Medical Applications for Radioisotopes
* Radiation is used to provide images of specific body tissues (compare X-rays).
• Iodine, used only by the thyroid gland, can be used to obtain an image of the thyroid gland because radioactive iodine will accumulate in this gland.
* Radiation is also used to destroy diseased and cancerous tissues.
• Emissions from radioactive sources can be used without injecting the patient with radioactive material.
Radioactivity and Radioisotopes
* Radiation comes in many different types and forms. Cosmic radiation is a natural radiation. It is a major source of radiation to which humans are exposed.
* Microwave radiation is an example of human-made radiation.
* When energy is given off spontaneously from the nucleus of an atom, it is called nuclear radiation.
* Isotopes that are not stable react by spontaneously emitting radiation from their nuclei. This process is called radioactive decay.
* Some isotopes are stable, i.e. they don’t decay.
Types of Radiation
* There are three main types of radiation, alpha, beta and gamma
Biological Effect of Radiation
* Radiation emissions are dangerous to living organisms because when emitted they interact with any atoms they contact.
* Alpha particles, beta particles, and gamma rays are known as ionizing radiation. When they come in contact with atoms, they cause the atoms to lose electrons, which leaves species that are reactive and unstable.
Alpha Decay
* The alpha particle is a product of alpha decay.
* To solve an alpha particle decay equation, remember that the mass and atomic numbers must be equal on both sides of the equation. Then you can look at the periodic table for the element that has the appropriate atomic number.
 Beta Decay
Beta Decay
* The high energy electron is emitted from an isotope during beta decay. Remember this particle has no mass and a negative charge.
* The product isotope will have the same mass as the reactant, but its atomic number will increase by 1 since the beta particle is negatively charged.
Nuclear Equations and Radioactive Decay
* The general form of a nuclear decay equation is:
Radioactive nucleus undergoing decay ?
new nucleus formed + radiation emitted
* Uranium-238, a radioactive isotope, emits alpha particles when it undergoes radioactive decay. The nuclear equation for this type of decay is:
Mass and charge are conserved
Gamma Decay
* Gamma decay emits only energy (no mass) and will not result in a change of the mass number or atomic number of the product. Remember gamma rays have no mass and no charge.
Half-Life
* Each radioactive isotope emits its radiation at a different rate.
* Emission of radiation can be measure by its half-life.
* The half-life of an isotope is the time it takes for 50% of the atoms in a radioactive sample to decay.
* Naturally occurring radioisotopes tend to have long half-lives. Radioisotopes used in medicine were chosen to have shorter half-lives to allow radioactivity to be quickly eliminated from the body.
Half-Life
Working with half-life
* Radioactive iodine-131 has a half-life of 8 days. If a dose of 200 µC is given to a patient, how much activity is left after 32 days?
Measuring Radioactivity
* The activity of a radioactive sample is measured in disintegrations/second.
* The unit of measuring disintegrations is called the curie (Ci).
* The activity of a radioactive isotope defines how quickly it emits radiation. A curie is a unit of activity equal to 3.7 x 101 disintegrations/second.
 States of matter
States of matter
* (s): solid, has definite shape and volume.
Atoms in a solid are tightly packed (touch their neighbors).
* (l): liquid, has a definite volume, but its shape changes depending on the container it is in.
Atoms in a liquid touch their neighbors, but can move large distances.
* (g): gas, has no definite volume or shape.
Atoms in a gas don’t touch their neighbors.
Why study gases?
* Biology: breathing is gas exchange
* Chemistry: Important in figuring out that matter consists of atoms
* Convenient for measuring pressure and temperature
* Shows that matter is made of particles
Gases or not?
* Gas (gasoline) at the gas station?
(carburetor: turns it into gas mixture with air)
* Air?
* Steam (water vapor)?
* Helium?
* Gases are affected by changes in their environment such as increases or decreases in temperature and pressure.
Gases and Pressure
* Pressure is defined as a force exerted against a given area.
* Pressure measurements are expressed in units of pounds-force per square inch (psi), millimeters of mercury (mmHg), [and in science, Pascals]
Atmospheric pressure
 Millimeters of Mercury (mmHg)
Millimeters of Mercury (mmHg)
* measurement of the pressure as length of mercury column in Earth’s gravity that just counteracts pressure.
* atmospheric pressure at sea level is 760 mmHg.
Pressure and Volume—Boyle’s Law
* In the mid 1600s, Robert Boyle discovered that the volume of a gas decreases as the pressure on that gas increases. Temperature and amount of gas were not allowed to change.
Let’s try it!
* Boyle’s law states that the volume of a gas at constant temperature is inversely proportional to the pressure.
Working with Boyle’s Law
* What happens to a sample of gas of known volume and pressure if we make changes to the volume or the pressure while the temperature is held constant?
Example
* Assume you have a sealed cylinder with 0.500 L of gas at a pressure of 1 atm.
* If you increase the pressure to 2 atm, what would happen?
* If you increase the pressure to 3 atm, what would happen.
Measuring matter
* How much _____________
do you have?
Converting chemical amount to mass
* Molar mass M is the conversion factor between chemical amount n and mass m:
m = n x M
* example: I have a cup containing 10 moles of water. What is the mass of the water in the cup?
n(water) = 10 mol; M(water) = 18g/mol
 Where do we get the molar mass from?
Where do we get the molar mass from?
Periodic table:
Molar mass in g/mol
Why do the mass number and the molar mass in g/mol have the same value?
Mole is set such that the mass of one mole of protons is 1 g, and the mass of one mole of neutrons is 1 g.
Molar mass of He and CO2
* M(He): look it up
* M(CO2) = M(C) + 2*M(O) =
Electron Arrangements
* Electrons are bound because they are attracted by the positively charged nucleus
* The closer they are to the nucleus, the stronger bound they are (lower energy level)
* Because electrons repulse each other and because they are not at rest, they are at some distance to the nucleus
* Electrons occupy discrete energy levels number with n = 1 … 5 that “fit” a limited number of electrons [quantum physics explains why this is so]
Electron Arrangements
* Electrons occupy the lowest energy level, first which is closest to the nucleus.
* The maximum number of electrons in any energy level can be calculated by the formula 2*n*n, where n is the number of energy level.
* [n is called the main quantum number]
Electron Arrangements
* Hydrogen has 1 electron in the first shell
* Helium has 2 electrons - both in the first shell
* Lithium has 3 electrons - 2 in the first shell, and 1 in the second shell
* Beryllim has 4 electrons - 2 in the first shell, and 2 in the second shell
* Boron has 5 electrons - 2 in the first shell, and 3 in the second shell
* Carbon has 6 electrons - 2 in the first shell, and 4 in the second shell
* Nitrogen has 7 electrons - 2 in the first shell, and 5 in the second shell
* Oxygen has 8 electrons - 2 in the first shell, and 6 in the second shell
* Fluorine has 9 electrons - 2 in the first shell, and 7 in the second shell
* Neon has 10 electrons - 2 in the first shell, and 8 in the second shell
* Sodium has 11 electrons – 2 in the first, 8 in the second, and 1 in the third
Valence electrons are key to describing chemical bonds
 Noble gases
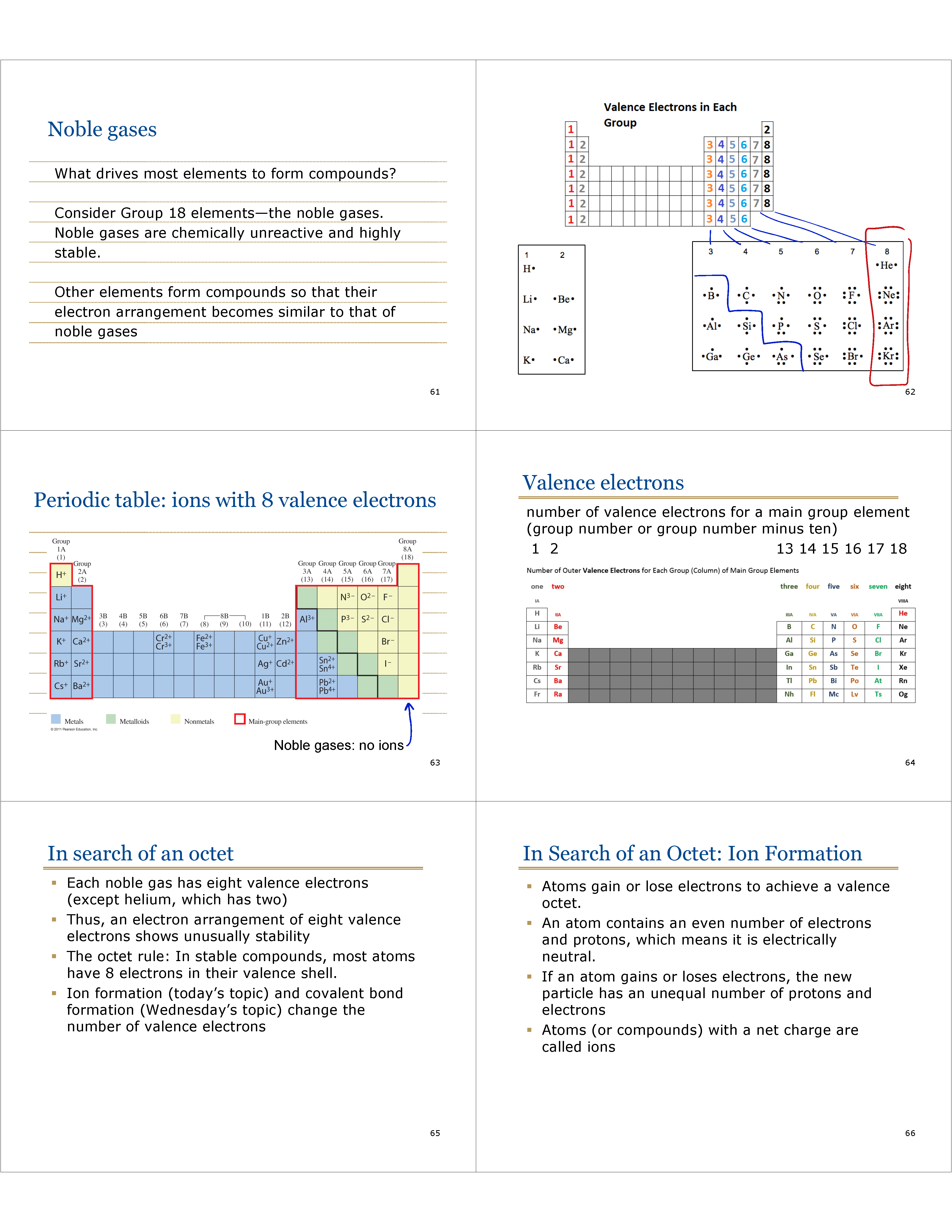
Noble gases
What drives most elements to form compounds?
Consider Group 18 elements—the noble gases.
Noble gases are chemically unreactive and highly stable.
Other elements form compounds so that their electron arrangement becomes similar to that of noble gases
Periodic table: ions with 8 valence electrons
Valence electrons
number of valence electrons for a main group element (group number or group number minus ten)
1 2 13 14 15 16 17 18
In search of an octet
* Each noble gas has eight valence electrons (except helium, which has two)
* Thus, an electron arrangement of eight valence electrons shows unusually stability
* The octet rule: In stable compounds, most atoms have 8 electrons in their valence shell.
* Ion formation (today’s topic) and covalent bond formation (Wednesday’s topic) change the number of valence electrons
In Search of an Octet: Ion Formation
* Atoms gain or lose electrons to achieve a valence octet.
* An atom contains an even number of electrons and protons, which means it is electrically neutral.
* If an atom gains or loses electrons, the new particle has an unequal number of protons and electrons
* Atoms (or compounds) with a net charge are called ions
 Anions have negative charge
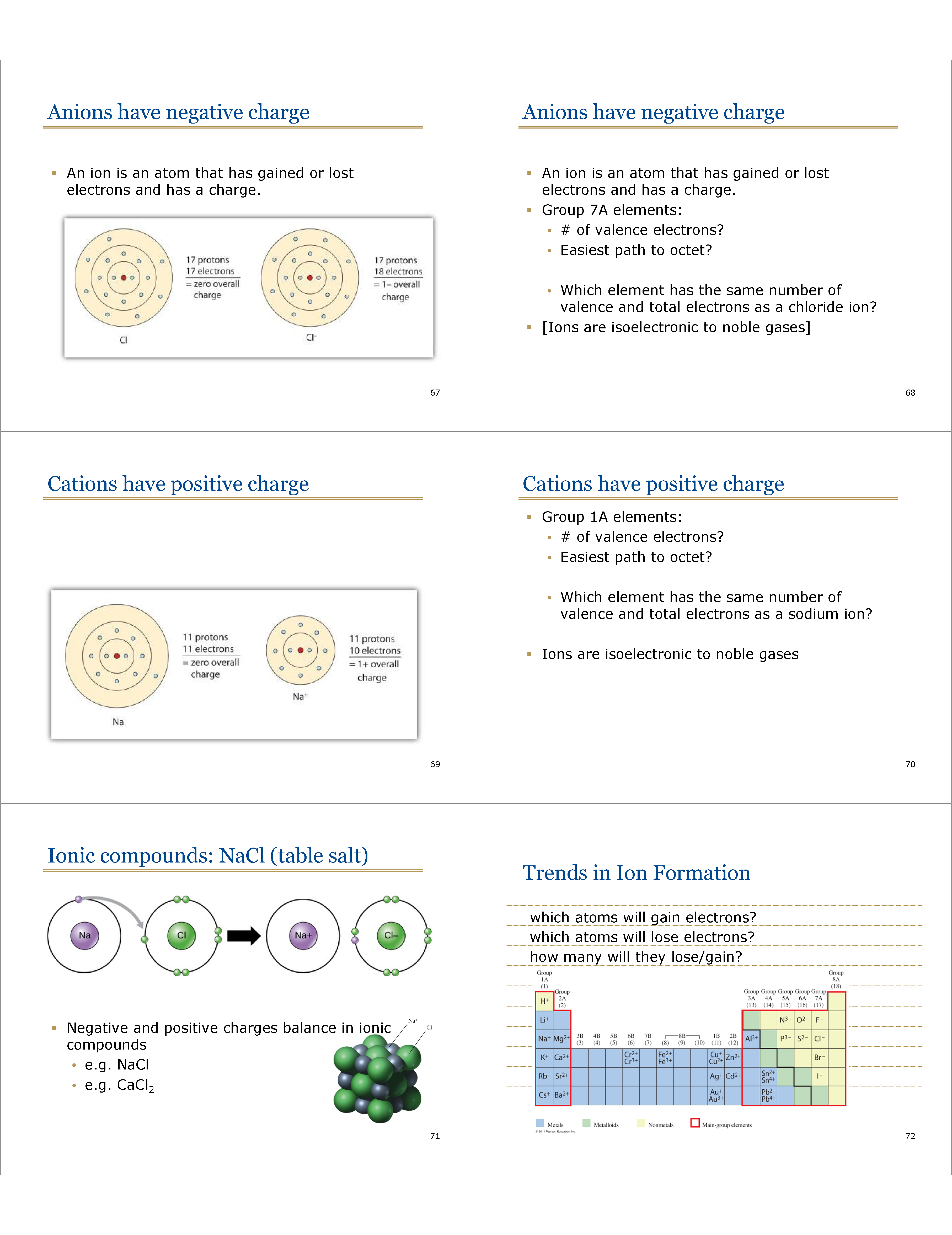
Anions have negative charge
* An ion is an atom that has gained or lost electrons and has a charge.
Anions have negative charge
* An ion is an atom that has gained or lost electrons and has a charge.
* Group 7A elements:
• # of valence electrons?
• Easiest path to octet?
• Which element has the same number of valence and total electrons as a chloride ion?
* [Ions are isoelectronic to noble gases]
Cations have positive charge
Cations have positive charge
* Group 1A elements:
• # of valence electrons?
• Easiest path to octet?
• Which element has the same number of valence and total electrons as a sodium ion?
* Ions are isoelectronic to noble gases
Ionic compounds: NaCl (table salt)
* Negative and positive charges balance in ionic compounds
• e.g. NaCl
• e.g. CaCl2
Trends in Ion Formation
which atoms will gain electrons?
which atoms will lose electrons?
how many will they lose/gain?
 Naming cations (metal ions)
Naming cations (metal ions)
To distinguish between an element and its ion, a different name is given to the ion
• To name metal ions, add the word ion to the name of the metal, so Na+ becomes the sodium ion. Same for K+, Mg2+, Ca2+
• Transition metals form more than one ion. A roman numeral in parentheses following the name of the metal indicates the charge. Fe2+ is iron(II) and Fe3+ is iron(III).
One special case: ammonium behaves like sodium and potassium ion, but is a polyatomic ion, NH4+
Naming anions
* nonmetal ions: the suffix –ide replaces the last few letters of the element’s name
• Cl: chlorine; Cl-: chloride ion
• O: oxygen; O2-: oxide ion
* polyatomic anions: most end in -ate
• e.g. carbonate, CO32-
• e.g. phosphate, PO43-
• e.g. sulfate, SO42-
• [–ite is used for names of related ions that have one less oxygen atom, e.g. sulfite]
Exceptions: hydroxide (OH-) and cyanide (CN-)
Memorize the ones in red
Covalent Bonding
* A covalent bond is formed by the sharing of electrons between two atoms (typically nonmetals)
• In ionic bonds, no electrons are shared
* A molecule is a set of atoms connected by covalent bonds. Some elements form molecules (e.g. oxygen, O2 and ozone, O3), but most molecules contain at least two types of atoms.
* Compounds made up of molecules are called covalent compounds or molecular compounds (as opposed to ionic compounds or metallic compounds)
Valence of atoms
* The rule of thumb in covalent bond formation is that the number of covalent bonds that an atom will form equals the number of electrons that it needs to complete its octet.
* The electron dot symbol (elemental symbol plus a dot for each valence electron) for an atom is used to discuss covalent bond formation.
• Write the symbol of the element.
• Place one valence electron on the top, bottom, right, and left of the element symbol.
• Continue placing the remaining valence electrons around the element symbol by forming electron pairs with the first four electrons.
Example: Chlorine is a diatomic molecule
* Chlorine needs only one electron to complete its octet, so it shares an electron from another chlorine atom.
* A bonding pair is a shared pair of electrons. The remaining valence electrons are known as the lone pair of electrons.
* The bonding and lone pair of electrons are shown for the newly formed molecule Cl2.
Covalent bonds with carbon
* Carbon, the element of life on Earth, has four unpaired valence electrons.
* Carbon can form
• four single bonds
• one double and two single bonds
• two double bonds (not shown, see CO2)
• one single bond and one triple bond
 Formulas and Structures of Molecules
Formulas and Structures of Molecules
* A molecular formula identifies all the components in a molecule of a covalent compound.
* Table sugar, sucrose (molecular formula C12H22O11) has 12 carbon atoms, 22 hydrogen atoms, and 11 oxygen atoms.
* Molecular formula only tells us the number of atoms in a molecule not how they are joined or what the structure is.
Lewis structure: atoms and bonds
* Consider methane, which has a molecular formula of CH4. It contains one carbon atom and four hydrogen atoms.
* To determine how atoms are connected, start by drawing the electron dot symbols for all the atoms involved.
* Then, share electrons by replacing two dots with a line. This representation is called a Lewis structure.
Naming Covalent Compounds
binary compound: covalent compound composed of only two elements.
Rules for naming binary compounds:
• Name the first element in the formula.
• Name the second element and change the ending to -ide.
• Designate the number of each element present using a Greek prefix (mono, di, tri…).
The number is important, e.g. CO (carbon monoxide) vs. CO2 (carbon dioxide)
9
• Shapes of molecules
• Polarity (how sticky is a molecule)
• Covalent bonds
• Lewis structures
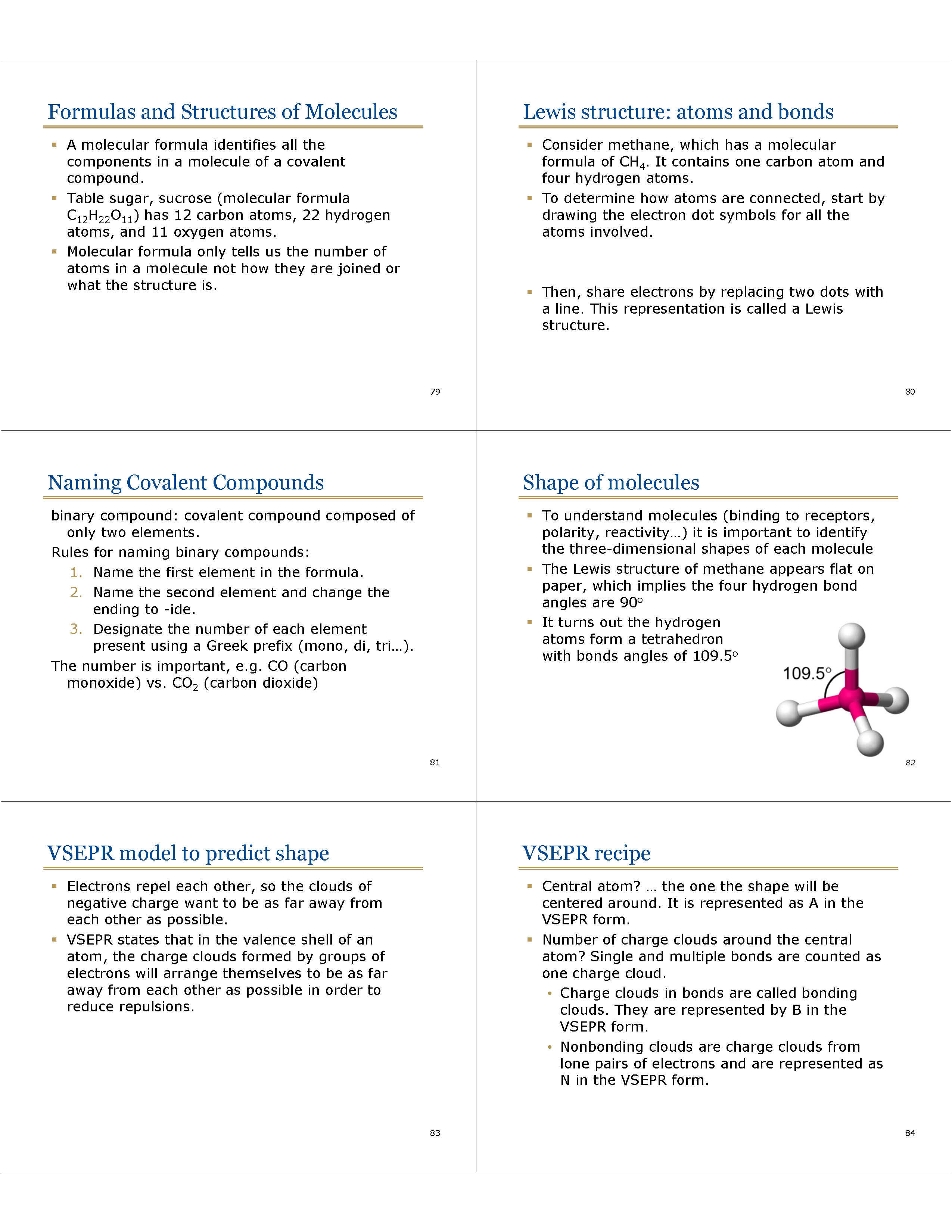
Shape of molecules
* To understand molecules (binding to receptors, polarity, reactivity…) it is important to identify the three-dimensional shapes of each molecule
* The Lewis structure of methane appears flat on paper, which implies the four hydrogen bond angles are 90o
* It turns out the hydrogen
atoms form a tetrahedron
with bonds angles of 109.5o
VSEPR model to predict shape
* Electrons repel each other, so the clouds of negative charge want to be as far away from each other as possible.
* VSEPR states that in the valence shell of an atom, the charge clouds formed by groups of electrons will arrange themselves to be as far away from each other as possible in order to reduce repulsions.
VSEPR recipe
* Central atom? … the one the shape will be centered around. It is represented as A in the VSEPR form.
* Number of charge clouds around the central atom? Single and multiple bonds are counted as one charge cloud.
• Charge clouds in bonds are called bonding clouds. They are represented by B in the VSEPR form.
• Nonbonding clouds are charge clouds from lone pairs of electrons and are represented as N in the VSEPR form.
Formaldehyde vs. Ammonia
Both have one central atom and three ligand atoms. Do they have the same shape?
 A catalog of shapes predicted by VSEPR
A catalog of shapes predicted by VSEPR
Polar vs. non-polar molecules
* In contrast to ions, molecules have a net charge of zero. However, if parts of a molecules are slightly positively charged and others slighlty negative charged, we call them polar.
* Polar molecules don’t mix with nonpolar molecules (e.g. water and oil), but within each group they often do mix (e.g. water and sugar)
* Ionic compounds dissolve in polar solvents (e.g. NaCl in water), but not in nonpolar solvent
* Small nonpolar molecules: often gases (e.g. O2)
Molecular Polarity
* Electrons are not share equally in covalent bonds. Instead, the more electronegative atom gets a larger share, i.e. a larger part of the negative charge
* Electronegativity is the ability of an atom to attract bonding electrons of a covalent bond to itself.
Partial charges
* The unequal sharing of electrons results in a so-called polar covalent bond.
* An uneven sharing of electrons in a polar covalent bond is denoted using the Greek symbol delta, ?, meaning partial.
Bonds: Ionic, polar covalent, non-polar
* The type of bond formed between two elements can be predicted by the difference between the electronegativities of the two elements.
• difference > 1.8: expect ionic
• difference < 1.8: expect covalent bond
• difference = 0: not polar
 Molecular Polarity
Molecular Polarity
* The interaction of electronegativity and molecular shape is the basis for determining if a molecule is polar or nonpolar.
* Both CO2 and H2O contain polar covalent bonds. However, carbon dioxide is linear and water is bent.
* As a result, CO2 is non-polar, H2O is polar
Rules for polarity (diatomic molecules)
• If the bond is nonpolar, the molecule is
nonpolar
• If the bond is polar, the molecule is polar.
Rules for polarity (more than 2 atoms)
• If there are no polar bonds, the molecule is
nonpolar (e.g. O3, ozone)
• If the central atom is surrounded by identical atoms and lacks nonbonding pairs of electrons, the molecule is nonpolar, even in the presence of polar bonds (e.g. CO2).
• If the central atom has nonbonding electrons, the molecule is polar (e.g. H2O or NH3).
• If the central atom is surrounded by atoms that are not all the same, the molecule is polar. (e.g. CHCl3, chloroform)

